|
Glossary
EVISA is providing a list of terms used in the area of speciation and fractionation analysis. Since speciation analysis is a field of analytical chemistry that is specified by a pronounced interdisciplinary cooperation between different sciences such as biochemistry, medicine, biology, environmental sciences, nutritional sciences and material sciences its terminology is a complex mixture of terms used in all these.
You may search for a term or browse the glossary alphabetically.
(In case that you cannot find the term you may consult more special glossaries or handbooks about nomenclature. For more details please consult EVISA's Link pages related to terminology,
|
|
Cacodylic acid (also called Dimethylarsinic acid) has the chemical formula (CH3)2AsO2H.
|
| |
|
|
Cadmium is a chemical element in the periodic table that has the symbol Cd and atomic number 48. A relatively rare, soft, bluish-white, transition metal, cadmium is known to cause cancer and occurs with zinc ores. Cadmium is used largely in batteries and pigments, for example in plastic products.
|
| |
|
|
The set of operations which establish, under specified conditions, the relationship between values indicated by a measuring instrument or measuring system and the corresponding known values of the measurand.
|
| |
|
Plot of analytical response vs. known mass or concentration of analyte.
|
| |
|
|
A Ca2+ binding protein involved in metabolic regulation.
|
| |
|
Any one of a group of diseases that occur when cells in the body become
abnormal and grow or multiply out of control.
Source: ATSDR Glossary of Terms
|
| |
|
A theoretical risk for getting cancer if exposed to a substance every
day for 70 years (a lifetime exposure). The true risk might be lower.
Source: ATSDR Glossary of Terms
|
| |
|
|
Refers to chromatography columns with inner diameters less than 0.5 mm.
|
| |
|
|
Capillary electrochromatography (CEC) is a hybrid technique in which capillary columns are packed with chromatographic sorbents and electroosmotic flow rather than pressure moves mobile phase through the column; technique has the surfacemediated selectivity potential of HPLC and the high efficiency of capillary electrophoresis (CE).
|
| |
|
|
Electrophoretic separation technique performed in a small fused silica capillary.
|
| |
|
|
The main separation mechanism in CGE is based on differences in solute size as analytes migrate through the pores of the gel-filled column. Gels are potentially useful for electrophoretic separations mainly because they permit separation based on 'molecular sieving'. They serve as anti-convective media, minimize solute diffusion, which contributes to zone broadening, prevent solute adsorption to the capillary walls and they help to eliminate electroosmosis.
|
| |
|
|
The main feature of CITP is that it is performed in a discontinuous buffer system. Sample components condense between leading and terminating constituents, producing a steady-state migrating configuration composed of conservative sample zones. This mode of operation is therefore different from other modes of capillary electrophoresis, eg CZE, which are normally carried out in a uniform carrier buffer and is characterized by sample zones which continuously change shape and relative position. In the case of a typical CZE separation, the electropherogram obtained contains sample peaks similar to those obtained in chromatographic separations, whereas in the case of CITP, the isotachopherogram obtained contains a series of steps, with each step representing an analyte zone. Unlike in other CE modes, where the amount of sample present can be determined from the area under the peaks as in chromatography, quantitation in CITP is mainly based on the measured zone length which is proportional to the amount of sample present.
|
| |
|
|
Capillary zone electrophoresis (CZE) is an electrophoretic separation technique using an electrolyte-filled capillary. When an electric field is applied across the capillary, ionic species migrate towards the oppositely charged electrode with a linear velocity depending on the electrophoretic mobility of the species in the electrolyte. Because of their solvation, the ion movement drags the bulk solution in the capillary towards the cathode resulting in an electroosmotic flow.
|
| |
|
Carbasone was introduced in 1931 as one of the antiprotozoal organoarsenicals against infections with Trichomonas vaginalis and Entamoeba histolytica. Today it is in use as a antiparasitic food additive for poultry and swine.
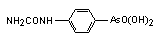 Synonyms for Carbasone: N-carbamoylarsanilic acid, [4-[aminocarbonyl-amino]phenyl] arsonic acid, Amabevan, Ameban, Amibiarson, Arsambide, Carb-O-Sep, Histocarb, Fenarsone, Leucarsone, Aminarsone, Amebarsone Synonyms for Carbasone: N-carbamoylarsanilic acid, [4-[aminocarbonyl-amino]phenyl] arsonic acid, Amabevan, Ameban, Amibiarson, Arsambide, Carb-O-Sep, Histocarb, Fenarsone, Leucarsone, Aminarsone, Amebarsone
|
| |
|
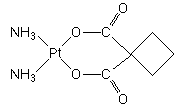 A "second generation" platinum metallodrug effective in cancer chemotherapy that has been approved by the FDA for the treatment of ovarian cancers in 1989. Carboplatin is less toxic than the "first generation" antitumor drug, cisplatin.
IUPAC name: cis-diammine(cyclobutane-1,1-dicarboxylato)platinum(II).
|
| |
|
A substance or agent that produces or incites cancerous growth.
|
| |
|
Cancer-causing; many agents that are carcinogenic are mutagens (agens that increase the occurence of mutation),
|
| |
|
|
a process by which materials are carried into a reaction mixture to which they do not belong. These materials can be either parts of a specimen, or reagents including the diluent or wash solution. In such cases, carry-over means the transfer of material (specimen or reagents) from one container, or from one reaction mixture, to another one. It can be either unidirectional or bidirectional in a series of specimens or assays. The term carry-over effect is used for carry-over from specimen to specimen.
|
| |
|
|
- a device that uses
a series of impaction stages with decreasing particle cut size so that
particles can be separated into fractions having relatively narrow intervals of
aerodynamic diameter; used for measuring the aerodynamic size
distribution of an aerosol
|
| |
|
A medical or epidemiologic evaluation of one person or a small group of
people to gather information about specific health conditions and past
exposures.
Source: ATSDR Glossary of Terms
|
| |
|
A study that compares exposures of people who have a disease or condition
(cases) with people who do not have the disease or condition (controls).
Exposures that are more common among the cases may be considered as possible
risk factors for the disease.
Source: ATSDR Glossary of Terms
|
| |
|
|
The electrode where reduction occurs in an electrochemical cell. It is the
negative electrode in an electrolytic cell, while it is the positive electrode
in a galvanic cell. The current on the cathode is considered a negative current
according to international convention; however, in electroanalytical chemistry
the cathodic current is often considered positive. Contrast with anode.
|
| |
|
|
An ion having a positive charge. Atoms of metals, in solution, become cations.
|
| |
|
|
The ability of a solid substance (especially clay minerals) to adsorb cations. The cation exchange capacity of a material represents the total negative charge on the surface of the material and is generally expressed in milliequivalents per 100 g of material (compare with anion exchange capacity).
|
| |
|
the form of ion-exchange chromatography that uses resins or packing with functional groups that can separate cations. A sulfonic acid would be an example of a strong cation-exchange group; a carboxylic acid would be a weak cation-exchange group.
|
| |
|
|
Multi-pixel detector in which potential bias supplied to adjacent portions of a semiconductor create wells in which electrons may be stored during exposure and between which electrons may be transferred during readout.
|
| |
|
|
separation technique based on particle density. By varying the rotation speed and centrifugation times, good separations of suspended and colloidfal particles can be achieved.
|
| |
|
A plasma torch where either (or all) the sample injector, inner tube, or outer tube is made of a ceramic material. Typically has a longer lifetime than a traditional quartz torch, especially when hydrofluoric acid has been used for sample preparation.
|
| |
|
|
reference material with a certificate in which some values are certified by a procedure assuring its traceability to the unit in which values are expressed. The certified value is given with an uncertainty at a stated confidence level.
|
| |
|
|
Ceruloplasmin is a large protein with a molecular weight of 132,000 daltons and the main copper-binding protein in blood plasma. Each molecule will bind six atoms of copper, containing type 1, type 2 and type 3 copper centers, where the type 2 and type 3 are close together, forming a trinuclear copper cluster. Low values of ceruloplasmin are found in Wilson's disease, malnutrition and Menke's kinky hair syndrome. Elevated levels are found during pregnancy, estrogen or anti-seizure drug use, infections, tissue necrosis and trauma.
|
| |
|
|
Code of Federal Regulations. A collection of the regulations that
have been promulgated under U.S. law.
|
| |
|
|
Also called the Challenger scheme, which was proposed by Frederick Challenger. The mechanism is a series of metabolic reduction and oxidative methylation reactions that begin with the reduction of inorganic arsenate to inorganic arsenite and ultimately ends with the formation of trimethylarsine.
|
| |
|
|
A detector used in ICP-MS to convert ions into electrical pulses using the principle of multiplication of electrons via a potential gradient inside a sealed tube.
|
| |
|
Proteins that help in folding proteins correctly and discourages incorrect folding. Metallochaperones assists in
the delivery of metal ions to target proteins or compartments.
|
| |
|
|
The charge state is the imbalance between the number of
protons (in the nuclei of the atoms) and the number of electrons that a
molecular moiety possesses. If the moiety possesses more protons than
electrons, its charge state is positive; if it possesses more electrons
than protons its charge state is negative.
|
| |
|
Charge stripping is a process by which atomic ions are transformed into a higher charge state, thereby changing their mass-to-charge ratios. Different cross sections for charge stripping of isobaric atomic ions allow their differentiation and subsequent trace level analysis.
  
|
| |
|
Sometimes referred to as “charge exchange.” This is one of the ion–molecule reaction mechanisms that take place in a collision/reaction cell. It involves the transfer of a positive charge from the interfering ion to the reaction gas molecule, forming a neutral atom that is not seen by the mass analyzer. An example of this kind of reaction is
H2 + Ar+ = Ar + H2+
|
| |
|
|
A QC sample prepared independently of calibration standards, analyzed exactly like the samples and used to estimate analytical precision and indicate bias due to calibration.
|
| |
|
The interaction of a metal ion with a multidendate ligand involving coordination with than one ligand atom. The complex formed has ring structure and is highly stable.
|
| |
|
|
An ion exchange resin in which the counter-ions are retained by a chelate functional group on the resin.
|
| |
|
|
Chelation involves coordination of more than one sigma-electron pair
donor group from the same ligand to the same central atom. The number
of coordinating groups in a single chelating ligand is indicated by the
adjectives didentate, tridentate, tetradentate, etc..
|
| |
|
The judicious use of chelating (metal binding) agents for the removal
of toxic amounts of metal ions from living organisms. The metal ions
are sequestered by the chelating agents and are rendered harmless or
excreted. Chelating agents such as 2,3-dimercaptopropan-1-ol,
ethylenediaminetetraacetic acid, desferrioxamine and D-penicillamine
have been used effectively in chelation therapy for arsenic, lead, iron
and copper, respectively.
|
| |
|
|
A commercially available chelating resin, having a styrene-divinylbenzene copolymer incorporating iminodiacetate chelating groups.
|
| |
|
Chemi-ionization is the reaction of an atom or molecule with an internally excited atom or molecule to form an ion.For example internally excited G reacts with M to form the molecular ion:
G* + M → M+ + e− + G. + e− + G.
|
| |
|
|
those interferences that occur due to chemical processes which take place in flames. These include formation of compounds of low volatility, dissociation equilibria, and ionization equlibria.
|
| |
|
|
CI is a relatively soft
ionization method used for mass spectrometry. In positive-ion mode, ionization is
effected by ion-molecule reactions occurring between ions generated by
electron ionization (EI) of a reagent gas and the neutral molecules of the
analyte.
|
| |
|
|
The process of chemically modifying the sample in electrothermal atomic absorption spectrometry (ETAAS) or electrothermal vaporization (ETV) ICP-MS work to separate the analyte from the matrix. Also refer to chemical modifier and electrothermal vaporization (ETV).
|
| |
|
|
A chemical substance that is added to the sample in an electrothermal vaporizer to change the volatility of the analyte or matrix. Typically the modifier is added before the drying and ashing stages of the heating program to separate the vaporization of the analyte from the potential interferences of the matrix components.
|
| |
|
|
Background signals observed in the mass spectrum originating either from the presence of impurities in the sample or in the sample matrix, or from the presence of extraneous compounds in the absence of analyte.
|
| |
|
|
small shift in binding energy due to the chemical environment the atom is in.
|
| |
|
|
chemical elements: specific form of an elelemt defined as to isotopic composition, electronic or oxidation state, and/or complex or molecular structure
|
| |
|
|
Type of speciation analysis, providing information about the oxidation state and coordination environment of an element by using a microbeam or X-ray technique such as XPS or XAFS.
|
| |
|
|
Electrode with controlled modification of the electrode surface in order to enhance the selectivity and sensitivity.
|
| |
|
|
A chemical adsorption process in which weak chemical bonds are formed between gas or liquid molecules and a solid surface.
|
| |
|
|
In drug discovery, chemical genomics, or chemogenomics, involves screening chemical compounds against genes or gene products, such as proteins or other targets. Through this functional analysis, researchers hope to elicit gene response, tease out drug candidates, and identify and validate therapeutic targets.
|
| |
|
|
Chemometrics is the chemical discipline that uses mathematical and statistical methods to design or select optimal procedures and experiments, and to provide maximum chemical information by analysing chemical data.
|
| |
|
Chiral HPLC columns are made by immobilising single enantiomers onto
the stationary phase. Resolution relies on the formation of transient
diastereoisomers on the surface of the column packing. The compound
which forms the most stable diastereoisomer will be most retained,
whereas the opposite enantiomer will form a less stable diastereoisomer
and will elute first.
The forces that lead to this interaction are very weak and require
careful optimisation by adjustment of the mobile phase and temperature
to maximise selectivity. Typically a free energy of interaction
difference of only 0.03 kJ/mol between the enantiomers and the
stationary phase will lead to resolution.
The effect of temperature is important in chiral HPLC. Lower
temperature will increase chiral recognition, but as it alters the
kinetics of mass transfer, it may actually make the chromatography
worse by broadening peaks. There is often an optimum temperature for a
separation and knowledge of this gives the analyst another factor to
exploit in the method development process
The type of column used for separating a class of enantiomer is often
very specific, this combined with the high cost of chiral columns,
makes the choice of which column to use very critical.
|
| |
|
|
Chromated copper arsenate (CCA) is a chemical wood preservative containing the oxides of copper, chromium, and arsenate. CCA is used in pressure treated wood to protect wood from rotting due to insects and microbial agents.
|
| |
|
|
A plot of detector signal output or sample concentration versus time or elution volume during the chromatographic process.
|
| |
|
|
The physical method of separation in which the components to be
separated are distributed between two phases, one of which is stationary
while the other moves in a definite direction. Chromatography is a
widely used for the separation, identification, and determination of the
chemical components in complex mixtures.
|
| |
|
a hard, brittle, semi-gray metal. Uses includes alloys and plating element on metal and plastic substrates for corrosion resistance. Protective coating for automotive and equipment accessories.
Hazard: Hexavalent chromium compounds are suspected of producing tumors of the lungs, and nasal cavity. Corrosive action on the skin and mucous membranes.
|
| |
|
 There are some indications that Chromium(III) functions as a co-factor to augment the action of insulin, and thus influences carbohydrate, protein, and fat metabolism (the biologic active chromium-containing species has not yet been identified). Since absorption of chromium(III) is very low, nutritional scientists have looked for a way to enhance its bioavailability. Picolinate is simply an organic ligand that enhances absorption of chromium from the digestive tract. There are some indications that Chromium(III) functions as a co-factor to augment the action of insulin, and thus influences carbohydrate, protein, and fat metabolism (the biologic active chromium-containing species has not yet been identified). Since absorption of chromium(III) is very low, nutritional scientists have looked for a way to enhance its bioavailability. Picolinate is simply an organic ligand that enhances absorption of chromium from the digestive tract.
|
| |
|
Low-molecular-weight chromium-binding substance (LMWCr; also known as chromodulin) is an oligopeptide that seems to transport chromium in the body. It consists of four amino acid residues; aspartate, cysteine, glutamate, and glycine, bonded with four (Cr3+) centers. It interacts with the insulin receptor, and has been confused with glucose tolerance factor .
Source: Wikipedia
|
| |
|
A molecule that absorbs UV or visible light.
|
| |
|
|
An electrochemical measuring technique used for electrochemical analysis or
for the determination of the kinetics and mechanism of electrode reactions. A
fast-rising potential pulse is enforced on the working electrode of an
electrochemical cell and the current flowing through this electrode is measured
as a function of time.
|
| |
|
|
An electrochemical measuring technique used for electrochemical analysis or
for the determination of the kinetics and mechanism of electrode reactions. A
fast-rising current pulse is enforced on the working electrode of an
electrochemical cell and the potential of this electrode is measured against a
reference electrode as a function of time. In an unstirred solution, the potential will rise to the electrode potential
of the reaction requiring the least amount of energy to proceed, and it will
increase in time due to the concentration overpotential developing as the
concentration of the reactant is exhausted at the electrode surface. If the
current is larger than the limiting current, eventually the diffusional process
will not be able to provide the required flux for the current, and the electrode
potential will sharply rise (at the transition time) until it reaches the
electrode potential of the next available reaction in the solution, and so on.
|
| |
|
|
The treatment of certain diseases, especially rheumatoid arthritis, with
gold compounds.
|
| |
|
Collisionally Induced Dissociation (CID) and Electron Transfer Dissociation (ETD) are both key modes of dissociation within a collision or reaction cell, specifically important to MS-MS experiments with a mass spectrometer.
|
| |
|
|
HgS. Mercuric (mercury) sulphide. Most mercury mined comes from cinnabar ore
(rock containing cinnabar mineralization). The colour of cinnabar ranges from
cinnamon to brick and scarlet red, which explains its historic use as a pigment,
mainly in paints but also as a traditional colorant for foods.
|
| |
|
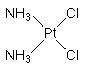 Cisplatin is a "first generation" antitumor drug highly effective in the chemotherapy of many forms of cancer. Thge metallodrug is most commonly used to treat testicular, bladder, lung, gullet (oesophagus), stomach and ovarian cancers. The water-soluble platinum complex, after hydrolysis, reacts with the nucleic acid bases of DNA to produce both intra and interstrand crosslinks. These crosslinks appear to impair replication and transcription of DNA. The cytotoxicity of cisplatin correlates with cellular arrest in the G2 phase of the cell cycle. IUPAC name: cis-diamminedichloroplatinum(II).
|
| |
|
Metal ion that combines preferentially with ligands containing ligating atoms that are the lightest
(smallest atomic number) of their periodic group.
See also borderline metal ion, class b metal ion, hard acid.
|
| |
|
Metal ion that combines preferentially with ligands containing ligating atoms other than the lightest (smallest atomic number) of their periodic group.
See also borderline metal ion, class a metal ion, hard acid.
|
| |
|
|
The Clean Air Mercury Rule (CAMR) is setting performance standards for new coal-fired power plants by nominally capping mercury emissions from new and existing plants at 38 tons per year (tpy) from 2010 to 2017 and 15 tpy in 2018 and thereafter; these down from 48.5 tpy in 1999. To implement the CAMR, 21 states with non-zero emissions adopted EPA’s new source performance standards and cap and trade program with little or no modification. By December 2007, 23 other states had proposed or adopted more stringent requirements; 16 states prohibited or restricted interstate trading of mercury emissions.
|
| |
|
A room in which the concentration of airborne particulate matter is controlled at specific limits to facilitate material operations with reduced risk for contamination. Clean rooms are classified according to the number of particles per volume of air to meet standards of cleanliness. Contaminants on surfaces and people entering and exiting the room also are controlled.
|
| |
|
|
analytical procedure for the removal of the matrix components (e.g. fats, proteins, high-boiling point hydrocarbons) that, if co-introduced on a chromatographic column would lead to their degradation or destruction. Clean-up is usually realized by low-resolution chromatographic separation with a mechanism different to that employed for the analytical separation (e.g. passing through a reversed phase of polar compounds to be separated by ion-exchange).
|
| |
|
Cloud point extraction (CPE), also called micelle-mediated extraction, is based on the phase separation behavior exhibited by aqueous solutions of some surfactants, usually nonionic, anionic, or zwitterionic. CPE has been recognized as an alternative approach to conventional liquid–liquid extraction because of a number of advantages: low cost, environmental safety, short analysis time, simultaneous pretreatment of a large number of samples is possible, and high recovery for a wide variety of analytes. The cloud point phenomenon occurs when aqueous solutions of surfactants at concentrations above the critical micelle concentration (CMC) associate to form molecular aggregates (micelles) upon modification of temperature or pressure, or introduction of a suitable additive,
|
| |
|
|
Codex
Alimentarius means "food code" and is the
compilation of all the Standards, Codes of Practice, Guidelines
and Recommendations of the Codex Alimentarius Commission (CAC).
|
| |
|
|
The Codex Alimentarius
Commission (CAC) is the highest international body on food standards.
The Commission is an intergovernmental body jointly sponsored by the Food and
Agriculture Organisation (FAO) and the World Health Organisation (WHO).
Membership is open to all Member Nations and Associate Members of
FAO/WHO, and currently comprises of over 169 countries. International
non-governmental organisations, such as consumer, academic or industry
bodies, may attend Codex meetings as observers. CAC's website can be reached at: http://www.codexalimentarius.net/.
|
| |
|
|
The relative standard deviation, i.e., the standard deviation expressed as a percentage of the mean [CV=100(s/x)].
|
| |
|
|
An injection that occurs at temperatures lower than the final oven temperature, usually at or below the solvent boiling point.
|
| |
|
|
A (inductively coupled argon) plasma operated under conditions to produce a much lower plasma temperature (typically by using a lower power and higher center gas flow rate) so that the ionization efficiency for ions with high ionization energies is greatly reduced.
|
| |
|
The fragmentation of an ion induced by collision with a neutral atom or molecule (in a collision cell).
|
| |
|
|
Chamber in the analysis tube of a mass spectrometer where accelerated ions and target gases collide in CID experiments.
|
| |
|
|
A region of relatively high pressure contained in an ion focussing device where fragmentation of an ion induced by collision with a neutral atom or molecule (CAD) takes place.
|
| |
|
|
A transmission hexapole or octapole collision cell to which an oscillating radio frequency potential is applied that is used for charge exchange neutralization of interfering ions in inductively coupled plasma mass spectrometry.
|
| |
|
In a collision between an ion and a neutral species, a portion of the ion translational energy is converted to internal energy. This internal energy causes dissociation of the ion into smaller fragment ions and can also cause changes in the ion charge. Collision-induced dissociation (CID) is also known as collisionally activated dissociation. Collision-induced dissociation is common in MS/MS experiments.
  
|
| |
|
|
In the quadrupole ion trap, ions are focussed towards the centre of the trap by collisional cooling with helium ions, where focussing forces are balanced by space charge. Ions with the lowest mass-to charge ratios are nearest the centre, with ions of higher mass arranged further out in layers, like the rings of an onion. These ions can be stored for long periods, providing improvements in mass selectivity and axial ejection, and enhancing process such as laser dissociation, since the ion cloud is more easily irradiated. Collisional focussing can also be carried out in other traps and focussing devices such as wave guides and hexapoles.
|
| |
|
|
The proprietary collisional reaction interface (CRI) used first in the Varian ICP-MS 820 and later in the Bruker ICP-MS Aurora M90 and Analytik Jena Plasmaquant is used to destroy interfering ions. These ions are removed by injecting a collisional gas (He), or a reactive gas (H2),
or a mixture of the two, directly into the plasma as it flows through
the skimmer cone and/or the sampler cone. Supplying the
reactive/collisional gas into the tip of the skimmer cone induces extra
collisions and reactions that destroy polyatomic ions in the passing
plasma. Fundamentally CRI is a mini- Collision/Reaction Cell installed
in front of the parabolic Ion Mirror optics.
|
| |
|
|
Material in the nanometer to micrometer size range whose characteristics and reactions are largely controlled by surface properties. Larger than most inorganic compounds, colloids will remain suspended indefinitely and will not normally diffuse through animal or vegetable membranes. The IUPAC definition of a colloid refers to "molecules or polymolecular particles" having at least one dimension in the range of 1-1000 nm.
|
| |
|
|
Typically a tube of sorts that contains the stationary phase of a chromatographic system.
|
| |
|
|
The normal background signal issuing from the chromatography column that reaches the detector in the absence of analyte. It results from the breakdown of the stationary phase. In practice, the total signal reaching the detector is often attributed to column bleed but it may also contain signals from the injector, detector and other components.
|
| |
|
|
A laboratory method that involves passing water, acids, or other leaching solutions through a stationary solid waste or another solid sample to model the dissolution of contaminants from the sample. The leaching process uses a plastic or glass column. The column is periodically recharged (usually at the top) with the leaching solution. The solution passes through the solids and is collected at the bottom of the column, usually filtered, and then analyzed for contaminants. Leachate collection may occur over days, weeks, or even months. Column leaching tests provide estimates of the types and concentrations of contaminants that are likely to mobilize from a wetted solid sample and move into the environment. Unlike batch leaching, no agitation of the column occurs and the system is open and more closely resembles subsurface conditions in many natural environments and contaminated sites. Although the procedures tend to be more expensive, time-consuming, and labor intensive than batch leaching, column leaching methods have the advantages of allowing observers to study longer term chemical interactions between solid samples and leachates, to note changes in the permeability of solid samples with time, and to evaluate how chemical reactions may change once more soluble compounds are flushed out of the solids (compare with leaching, batch leaching, leaching test, sequential batch leaching, and serial batch leaching).
|
| |
|
The particulate material packed inside the column; also called the stationary phase. This usually consists of silica- or polymer-based particles, often chemically bonded with a chemical function al group. For analytical work, 3µm or 5µm spherical particles are used; for semi-preparative, 10µm or larger spherical or irregular particles are favored. The most common packings are as follows:
- Octadecyl (ODS, C18) the most popular phase, used for reversed-phase HPLC
- Octyl (C8) more polar than C18, also a popular reversed-phase column
- TMS (methyl, C1) weakly retentive in reversed-phase
- Silica (Si) unbonded silica used in normal phase mode, popular for preparative LC.
- Phenyl, Cyano, Amino specialty phases used for specific applications.
|
| |
|
|
A chromatographic technique using multiple columns connected by switching valves for improved chromatographic separations or sample cleanup. Fractions from a primary column can be switched to two or more secondary columns, which in turn can be further diverted to additional columns or to detectors; sometimes called multidimensional chromatography.
|
| |
|
Any combination of a cation associated with molecules or anions
containing unshared pairs of electrons. The interaction can range from
purely electrostatic to that approaching covalent character.
With respect to a complex we further define:
- Metal Cation - The central metal atom in the complex
- Ligand(s) – the anions or molecules involved in forming the complex with the central metal cation
- Ligand Atom – the particular atom involved in the interaction with the central metal atom
|
| |
|
|
The chemical species (an ion or a compound) which will bond to a metal ion using lone pairs of electrons.
|
| |
|
|
A sample composed of several subsamples in order to represent a typical
situation such as a series of water samples taken over a given period
of time and weighted by flow rate or a soil sample that
consists of soil taken from various depths or various locations.
|
| |
|
|
(GC x GC): Twodimensional technique in which all compounds experience the selectivity of two columns connected in series by a retention modulation device, thereby generating much higher resolution than that attainable with any single column.
|
| |
|
|
Any one of a group of three quantities characterizing the composition of a mixture and defined as one of mass, amount of substance (chemical amount), or number divided by volume, giving, respectively, mass, amount (of substance), or number concentrations.
|
| |
|
- in biology: the extent to which an element or compound is concentrated in an organism (or specific tissue) over its environment (usually ambient water unless spercified as being related to sediment or diet); usually normalized on weight or volume bases
- in analytical chemistry: the extent to which a preconcentration technique concentrates the analyte over the concentration in the original sample.
|
| |
|
|
A concentric nebulizer is a pneumatic nebulizer in which the liquid
flows through a central capillary and dispersion gas flows through a
surrounding outer tube.
|
| |
|
An electroanalytical technique based upon the measurement of the conductivity
of an electrolyte solution. This technique is often used as a detection system in ion chromatography.
|
| |
|
|
the probability, in %, that a measurement result will lie within the confidence interval or between the confidence limits.
|
| |
|
|
The interval or range of values which will contain the population parameter with a specified probability.
|
| |
|
|
The confidence level is the probability of covering the expected value of an estimated parameter with an interval estimated for the parameter (symbol 1 – a). The confidence level can be expressed as a number between 0 and 1, or in percent. The complementary quantity is known as the significance level.
Comment: In some cases the confidence level is dictated by the needs of the situation.
|
| |
|
|
The extreme values or ‘end-values’ in a confidence interval.
|
| |
|
The shape of a molecule produced by the specific spatial arrangement of vthe units that compose it.
|
| |
|
Changing factor that can cause or prevent the outcome of interest, is not an intermediate variable, and is not associated with the factor under investigation: such a variable must be controlled in order to obtain an undistorted estimate of the effect of the study factor on risk.
[Last, 1988|] (IUPAC)
|
| |
|
|
Consecutive reaction monitoring (CRM) is the application of selected
reaction monitoring to sequential fragmentation in three or more stages
of mass spectrometry. The product ion from the first stage of mass spectrometry becomes the precursor ion for the second stage, and so on.
|
| |
|
|
Consensus value can be defined as the mean of‘ participants’ results on a test material distributed in a proficiency testing scheme, after outliers have been handled either by elimination or by the use of robust statistics.
|
| |
|
In trace analysis, this is the unintentional introduction of analyte(s) or other species which are not present in the original sample and which may cause an error in the determination. It can occur at any stage in the analysis. Quality assurance procedures, such as analyses of blanks or of reference
materials, are used to check for contamination problems.
|
| |
|
|
Continuous flow fast atom bombardment is a variant of the fast atom
bombardment ionization technique for mass spectrometry in which the analyte is dissolved in a liquid matrix that is
continuously supplied to the sample probe tip.
|
| |
|
|
Continuous flow matrix-assisted laser desorption ionization is a variant
of MALDI in which the analyte is dissolved in a liquid matrix that is
continuously supplied to the sample probe tip
|
| |
|
|
"A graphical method for evaluating
whether a process is or is not in a 'state of statistical control.' The
determinations are made through comparison of the values of some
statistical measure(s) for an ordered series of samples, or subgroups,
with control limits" [ASQ]. In healthcare laboratories, the
Levey-Jennings chart is commonly used to plot the result observed for a
stable control material versus time, usually the day or run number.
|
| |
|
|
"Limits on a control chart which are
used as criteria for signalling the need for action, or for judging
whether a set of data does or does not indicate a 'state of control'"
[ASQ]. Used here to refer to the defined limits or ranges of results
expected due to the random error of the method, and beyond which some
course of action should be taken. It is common in clinical laboratories
to use Levey-Jennings control charts with limits set as either the mean
plus or minus 2 standard deviations, or the mean plus or minus 3
standard deviations.
|
| |
|
|
A control solution
that is available, often commercially, liquid or lyophilized, and
packaged in aliquots that can be prepared and used individually.
|
| |
|
|
The
analytical results obtained for control solutions or control materials
(that are analyzed for purposes of quality control).
|
| |
|
|
Regulations which impose specific legal requirements for risk assessment wherever hazardous chemicals or biological agents are used.
|
| |
|
Surface that is held at high potential so that ions striking the surface produce electrons that are
subsequently detected.
|
| |
|
|
An operation mode for ICP-OES or ICP-MS used to reduce interferences. Under low temperature plasma (cool plasma) conditions, the formation of interferences such as Ar+, ArO+ and ArH+ is suppressed, allowing the detection of Ca+, Fe+ and K+ at the trace level. Typical RF power for cool plasma is 600-900 W.
|
| |
|
A spray chamber that is cooled below ambient tmperature in order to reduce the amount of solvent reaching the plasma discharge. Used for a variety of reasons, including reducing oxide species, minimizing solvent-based spectral interferences, and allowing the trouble-free aspiration of organic solvents otherwise surpassing the solvent load tolerance of the plasma.
|
| |
|
A coordination entity is composed of a central atom, usually that of a
metal, to which is attached a surrounding array of other atoms or group
of atoms, each of which is called a ligand. A coordination entity may
be a neutral molecule, a cation or an anion. The ligands may be viewed
as neutral or ionic entities that are bonded to an appropriately
charged central atom. It is standard practice to think of the ligand
atoms that are directly attached to the central atom as defining a
coordination polyhedron (tetrahedron, square plane, octahedron, etc.)
about the central atom. The coordination number is defined as being
equal to the number of sigma-bonds between ligands and the central
atom; this definition is not necessarily appropriate in all areas of
(coordination) chemistry. In a coordination formula, the central atom
is listed first. The formally anionic ligands appear next and they are
listed in alphabetic order according to the first symbols of their
formula. The neutral ligands follow, also in alphabetical order,
according to the same principle. The formula for the entire
coordination entity, whether charged or not, is enclosed in square
brackets. In a coordination name, the ligands are listed in
alphabetical order, without regard to charge, before the name of the
central atom. Numerical prefixes indicating the number of ligands are
not considered in determining that order. All anionic coordination
entities take the ending -ate, whereas no distinguishing termination is
used for cationic or neutral coordination entities.
|
| |
|
The number of ligands that the central metal atom will tend to bond
with in the formation of a complex. Defines the symmetry or geometry of
the metal complex.
|
| |
|
Copper is a chemical element that has the symbol Cu (Latin: cuprum) and atomic number 29. It is a ductile metal with excellent electrical conductivity, and finds extensive use as an electrical conductor, heat conductor, as a building material, and as a component of various alloys.
Copper is an essential nutrient to all high plants and animals. In animals, including humans, it is found primarily in the bloodstream, as a co-factor in various enzymes, and in copper-based pigments. In sufficient amounts, copper can be poisonous and even fatal to organisms.
|
| |
|
|
The
orally bioavailable copper salt of D-gluconic acid. In addition to its
roles as an enzyme cofactor for cytochrome C oxidase and superoxide
dismutase, copper forms complexes with the thiocarbamate disulfiram
(DSF) forming DSF-copper complexes, which enhances the DSF-mediated
inhibition of the 26S proteasome; proteasome inhibition may result in
inhibition of cellular protein degradation, cessation of cell cycle
progression, inhibition of cellular proliferation, and the induction of
apoptosis in susceptible tumor cell populations.
|
| |
|
A fungicide
that controls a wide range of fungal and bacterial diseases on fruit,
vegetables and ornamentals protecting the plant.
Formula: CuCl2 3 Cu(OH)2
|
| |
|
|
Occurs in solutions and refers to a minor or trace element (such as arsenic) adsorbing onto or absorbing within the developing or fresh precipitates of other chemical species. Although sometimes difficult to distinguish, sorption involves the incorporation of contaminants onto or within preexisting solids (sorbents), whereas coprecipitation occurs as or shortly after the host solids precipitate from solution, such as arsenic coprecipitating with iron (oxy)(hydr)oxides in acid mine drainage. Coprecipitation might also involve arsenic-bearing colloids or other fine-grained particles becoming trapped (absorbed) in the interiors of precipitating compounds (compare with precipitation and sorption).
|
| |
|
|
The cona discharge is an electrical discharge in the region
around the corona discharge needle that results in ionization of gas
molecules to form a chemical ionization (CI) plasma, which contains CI
reagent ions.
|
| |
|
|
An electroanalytical technique based upon the measurement of the amount of
electrical charge passed through the working electrode of an electrochemical
cell.
|
| |
|
|
The linking of two atoms, where valence electrons are more or less evenly shared between the two atoms (compare with ionic bond).
|
| |
|
|
Guideline value or a specified and defined concentration value; if the concentration of a substance is below or above this value, it will affect the classification of the object of study and any follow-up actions
|
| |
|
|
A calibration method that is used to correlate both pulse (low levels) and analog (high levels) signals in a dual-mode detector. This is possible because the analog and pulse outputs can be defined in identical terms (of incoming pulse counts per second) based on knowing the voltage at the first analog stage, the output current, and a conversion factor defined by the detection circuitry electronics. By carrying out a cross-calibration across the mass range, a dual-mode detector is capable of achieving approximately eight to nine orders of dynamic range in one simultaneous scan.
|
| |
|
|
A cross-flow nebulizer is a pneumatic nebulizer in which the dispersion
gas flows at a right angle with respect to the column of liquid emerging
from a capillary
|
| |
|
|
A stationary phase that includes cross-linked polymer chains. Usually, it also is bonded to the column inner wall. See also: bonded phase.
|
| |
|
|
collection of trace compounds from gaseous media by cocondensation with a major constituent (e.g. water vapour, CO2, N2, Ar) of the matrix
|
| |
|
|
Cold Vapor Atomic Absorption Spectroscopy - A technique for the analysis of mercury, whereby mercury is selectively chemically reduced to an elemental state and aerated from solution in a closed system. Absorption of the vapor at a given wavelength is a measure of the concentration of mercury in the sample.
|
| |
|
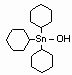 Cyhexatin is an organotin compound used as an insecticide. It inhibits oxidative phosphorylation at the site of dinitrophenol uncoupling, preventing the formation of the high-energy phosphate molecule adenosine triphosphate (ATP). Cyhexatin is an organotin compound used as an insecticide. It inhibits oxidative phosphorylation at the site of dinitrophenol uncoupling, preventing the formation of the high-energy phosphate molecule adenosine triphosphate (ATP).
IUPAC name: tricyclohexyltin hydroxide
CAS name: tricyclohexylhydroxystannane
Cas Reg. No.: 13121-70-5
Formula: C18H34OSn
|
| |
|
|
Iron-containing proteins important in cell respiration as catalysts of oxidation-reduction reactions.
|
| |
|
|
Conjugated proteins containing haem as the prosthetic group and associated with electron transport and with redox processes.
|
| |
|