|
Glossary
EVISA is providing a list of terms used in the area of speciation and fractionation analysis. Since speciation analysis is a field of analytical chemistry that is specified by a pronounced interdisciplinary cooperation between different sciences such as biochemistry, medicine, biology, environmental sciences, nutritional sciences and material sciences its terminology is a complex mixture of terms used in all these.
You may search for a term or browse the glossary alphabetically.
(In case that you cannot find the term you may consult more special glossaries or handbooks about nomenclature. For more details please consult EVISA's Link pages related to terminology,
|
The ever-increasing need to miniaturise the methods for handling and reacting substances (see nanotechnology) has led to the development of chips etched with channels and reservoirs which can be used to control the flows of separate fluids and their reactions with each other. The chips can be based on materials like silicon, quartz, glass, or silicon rubber. The chip can be used as a flow injection manifold. In MS, the fluid flows can be made to exit though a small aperture and, with the application of an appropriate electrical charge, be ionised by a nanoelectrospray process.
|
| |
|
|
The term has loosely been used to describe either a relatively unstable and transient chemical species or a relatively stable but reactive species. See also inert.
|
| |
|
|
Includes Hg(OH)2, HOHgCl, HgCl2, and weakly bound organo-complex forms
|
| |
|
|
Phosphorous that can be assimilated easily by biota.
|
| |
|
|
A known sample, usually prepared and certified by an outside agency, which is carried through the preparation and analysis procedures as if it were a sample. Replicate LCS results may be used to estimate precision and the difference between the mean of those results and the true value provides an indication of the magnitude of bias due to method error.
|
| |
|
A non-turbulent mode of fluid flow in which the fluid moves in a series of parallel layers, with no
interaction between the layers.
|
| |
|
|
An element which is any of the first series of f-block elements or inner transition elements commencing with lanthanum at atomic number 57 and ending with lutetium at atomic number 71.
|
| |
|
|
Lanthanum carbonate is a phosphate binder used to reduce the intestinal absorption of phosphates. It was approved as a medication (Fosrenol®, Shire Pharmaceuticals) to absorb excess phosphate in cases of end-stage renal failure (ESRD).
|
| |
|
|
This is a form of direct introduction of elements from solid samples in the ICP-MS. LA can be used for analysis of single spots from 2D-gel electrophoresis, tissue samples from food, animals, and mineral or archeological samples, etc.
|
| |
|
The production of a molecular species in the gas phase from a condensed phase by the application of a pulse of energy provided by a laser. For a fragile organic molecule to survive intact it is important that the process occurs in as short a time as possible. Lasers are successful as they can be fired in pulses of a few nanoseconds duration.
|
| |
|
Laser desorption/ionization mass spectrometry, particularly emphasizing spatially resolved composition information.
(see also: LAMMA)
|
| |
|
|
A microanalytical technique designed to detect and characterise the elemental composition of small sections of surfaces. A visible range laser is focussed, with a microscope, on the area of the section to be analysed. This is followed by a pulsed high-energy laser beam to desorb and ionise the elements present in that area. Areas as small as 0.5 micron can be defined. The ions so formed are analysed with a TOF mass spectrometer.
|
| |
|
|
Laser-Induced Breakdown Spectroscopy (LIBS) is a spectroscopic technique using a laser-generated plasma to ablate and excite a sample, which can initially be in solid, liquid, or gaseous form. Emission generated from the plasma is used to identify material constituents and can be used to identify, sort, and classify materials.
|
| |
|
|
a concentration of a substance that produces death in 50%
of a population of experimental animals after exposure for a period of
time which is usually specified (e.g. '96 hour LC50'). This term is
used when the substance exists in the organism's ambient environment at
the specified concentration (for example, fish in water in which the
substance is present at the specified concentration).
|
| |
|
|
a dose of a substance that produces death in 50% of a
population of experimental animals. It is usually expressed as
milligrams per kilogram (mg/kg) of body weight. This term is used when
the exposure pathway is by absorption of the specified dose.
|
| |
|
Liquid that has percolated through solid materials such as soil,
sediment or waste and has extracted dissolved or suspended materials
from it.
|
| |
|
Leaching is the process by which inorganic - , organic contaminants or radionuclides are released from the solid phase into the waterphase under the influence of mineral dissolution, desorption, complexation processes as affected by pH, redox, dissolved organic matter and (micro)biological activity. The process itself is universal, as any material exposed to contact with water will leach components from its surface or its interior depending on the porosity of the material considered.
Source: Surface and Aqueous Geochemistry Group, Stanford, USA
|
| |
|
Refers to laboratory procedures that use water, acids, bases, organic solvents, salt solutions, or other liquids to estimate the types and concentrations of contaminants that will dissolve out of a solid waste or another solid sample. Leaching procedures are useful in predicting whether the dumping or burial
of a solid material could potentially release problematic levels of contaminants into groundwater, surface waters, or other natural environments. Leaching procedures include batch, sequential batch, serial batch, and column methods. Some batch leaching tests, such as the toxicity characteristic leaching procedure (TCLP), are used in regulations to identify solid and liquid wastes for the toxicity characteristic of hazardousness.
|
| |
|
|
Lead is a chemical element in the periodic table that has the symbol Pb (Latin: plumbum) and atomic number 82. A soft, heavy, toxic and malleable poor metal, lead is bluish white when freshly cut but tarnishes to dull gray when exposed to air. Lead is used in building construction, lead-acid batteries, bullets and shot, and is part of solder, pewter, and fusible alloys. Lead has the highest atomic number of all stable elements - although the next element, bismuth, has a half life so long (longer than the estimated age of the universe) it can be considered stable. Like mercury, another heavy metal, lead is a potent neurotoxin which accumulates in soft tissues and bone over time.
|
| |
|
|
Classified as a blister agent (vesicant), Lewisite is named after the American military scientist, W. Lee Lewis, who produced this organoarsenic compound as a prototype chemical warfare (CW) agent in 1918. Because it was developed so late in World War I, Lewisite was never used in that conflict. The only military use of Lewisite known probably occurred in China during the Sino-Japanese conflict (ca. 1937-1942). Unlike mustard, which has delayed onset of clinical symptoms, the extreme irritation to eyes and skin begin almost immediately, with redness and blisters forming hours later. Significant exposure to Lewisite can cause blindness. Because of the rapid onset of pain, however, most exposures to Lewisite will result in less damage to the eyes as victims will attempt to avoid further contact by closing eyelids and avoiding the area. Lewisite, in its pure form, is an oily, colorless liquid, with no detectable odor. However, some have described impure batches of Lewisite as being of amber or dark brown color and having an odor of geraniums.
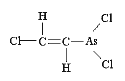 Depending on environmental conditions, Lewisite is a semi-persistent agent, and can penetrate a variety of rubber products, including those used in protective garments. Liquid at low temperatures (well below freezing), Lewisite was sometimes employed to mix with mustard to keep both CW agents solvent for use in winter conditions. Concentrations of Lewisite that can cause injury and death closely resemble those of mustard agent (also a vesicant). The median lethal concentration (LCt50) for Lewisite is about 1.5 grams-min/m3 for inhalation, and the median lethal dose (LD50) on the skin is estimated at 30mg/kg, or about 2.5 grams for an adult male of 180 pounds. The chemical formulation of Lewisite is relatively simple, and many countries, including those in the developing world, are capable of producing it in militarily significant quantities. |
| |
|
Life Sciences encompasses a range of scientific disciplines that center on the study of life in all its forms, from animals to plants and microorganisms. These include microbiology, biochemistry, genetics, medicine, pharmacology, agriculture, botany, zoology, food and nutrition.
|
| |
|
|
The molecule, ion, or group bound to the central atom in a chelate or a coordination compound.
|
| |
|
|
The lowest concentration of analyte that can be (qualitatively) detected to be statistically different from the blank signal at a specified level of confidence.
Not to be confused with 'limit of determination'.
|
| |
|
For an individual analytical procedure, this is the lowest amount of an analyte in a sample which can be quantitatively determined with suitable uncertainty. It may also be referred to as the
limit of determination. The limit of quantitation can be taken as 10 × ‘the signal-to-noise ratio’, assuming a ‘zero blank’.
|
| |
|
Study incorporating the study of all aspects of inland freshwater habitats including lakes, ponds, rivers,
streams, swamps, wetlands, groundwaters, and reservoirs that make up inland water systems
|
| |
|
|
The concentration range over which the analytical working calibration curve remains linear.
|
| |
|
|
The concentration range over which the instrument calibration curve remains linear.
|
| |
|
|
A statistical technique for estimating the best linear relationship
between two variables. The estimated line has the property that the sum
of the squares of the deviations from the line is a minimum, hence the
name least-squares analysis. This statistical technique is commonly
applied to the data from a comparison of methods experiment, taking the
test method values as the y-variable and the comparison method values as
the xvariable. The statistics calculated usually include the slope (b),
y-intercept (a), and standard deviation about the regression line, also
termed the standard error of the regression line (sy/x) and also called
the standard deviation of residuals (sres). These statistics provide
information about the proportional, constant, and random errors between
the methods, respectively.
|
| |
|
|
This defines the ability of the method to obtain test results proportional to the concentration of analyte.
|
| |
|
In a sector instrument with both magnetic and electric sectors, a linked scan is an experiment in which both sector field values (the magnetic and the electric sector values) are changed simultaneously so that ion mass- and charge-changing reactions that occur after the ion source, but before the sectors, or in the field-free region between the sectors, can be recorded in a mass spectrum.
  
|
| |
|
|
molecular entities (or part of molecular entitites) having a tendency to dissolve in fat-like (e.g. hydrocarbon) solvents
|
| |
|
Analytical technique used to separate mixtures of substances based oin the differential distribution of vthe substances between a stationary phase (material such as silica gel or silicic acid, usually contained in a column, tube, or capillary) and a liquid mobile phase (a medium that carries the sample through the stationary phase).
|
| |
|
LC-MS is a hyphenated technique in which a liquid chromatograph is
coupled with a mass spectrometric analyzer. For coupling the LC
operated at atmospheric pressure with the high vacuum mass analyzer a
lot of different interfaces have been designed ranging from moving belt
over continuous-flow FAB, frit-FAB to ESI and APCI, with ESI and APCI
being the most successful interfaces.
|
| |
|
|
A method of extracting a desired component from a liquid mixture by bringing the solution into contact with a second liquid, the solvent, in which the component is also soluble, and which is immiscible with the first liquid, or nearly so. Separation is based on
different solubilities of the solute in the two phases. The
liquid-liquid extraction is a relatively gentle separation process and
is therefore suitable for unstable molecules
|
| |
|
|
The carbonate salt of lithium, a soft alkali metal, with antimanic and
hematopoietic activities. Lithium interferes with transmembrane sodium
exchange in nerve cells by affecting sodium, potassium-stimulated
adenosine triphosphatase (Na+, K+-ATPase); alters the release of
neurotransmitters; affects cyclic adenosine monophosphate (cAMP)
concentrations; and blocks inositol metabolism resulting in depletion
of cellular inositol and inhibition of phospholipase C-mediated signal
transduction. The exact mechanism through which lithium exerts its
mood-stabilizing effect has not been established. In addition, lithium
stimulates granulocytopoiesis and appears to increase the level of
pluripotent hematopoietic stem cells by stimulating the release of
hematopoietic cytokines and/or directly acting on hematopoietic stem
cells.
|
| |
|
|
Lixiviation (or leaching) is the term for defining a process that allows removal of some components from a solid. Instead of this specific name, many scientists prefer the most generic term "extraction" (e.g. , supercritical fluid extraction or SFE, microwave-assisted extraction or MAE etc, accelerated solvent extraction or ASE), which is much less self-explanatory. The advantage of this process, whatever its name, in comparison to digestion or total dissolution is, that the leachate or "extract" contains less-potential interferents and so, the sub-sequent clean-up and separation steps can be either avoided or minimized. In general, this process can also be assisted by the same type of energy as dissolution, i.e. ultrasound, high pressure and temperature, and microwaves.
|
| |
|
 Lobaplatin (D-19466; 1,2-diammino-methyl-cyclobutaneplatinum(II)-lactate) is a new anticancer agent and a representative of the third-generation platinum compounds. The metallodrug lobaplatin consists of a nearly 50%/50% mixture of two diastereoisomers: (a) the SSS configuration (LP-D1); and (b) the RRS configuration (LP-D2) (see left.) . The compound has shown antitumor activity in human lung, gastric, testicular, and ovarian cancer xenografts, with incomplete cross-resistance to cisplatin in vitro and in vivo. Lobaplatin (D-19466; 1,2-diammino-methyl-cyclobutaneplatinum(II)-lactate) is a new anticancer agent and a representative of the third-generation platinum compounds. The metallodrug lobaplatin consists of a nearly 50%/50% mixture of two diastereoisomers: (a) the SSS configuration (LP-D1); and (b) the RRS configuration (LP-D2) (see left.) . The compound has shown antitumor activity in human lung, gastric, testicular, and ovarian cancer xenografts, with incomplete cross-resistance to cisplatin in vitro and in vivo.
|
| |
|
Over a period of time, the m/z calibration of some mass analysers can drift. This can be corrected by introducing and detecting an ion of known m/z value and correcting the electrical parameters of
the analyser to keep this ion in focus. In common use with magnetic sector and TOF analysers
especially when accurate mass measurement is being obtained.
|
| |
|
|
"The London Arsenic Group brings together expertise from the fields
of sedimentary geochemistry, hydrochemistry, environmental mineralogy
and analytical geochemistry. We seek to understand the source,
mobility, and fate, of arsenic in the environment. We exist to bring a
multi-disciplinary approach to this issue and provide a focus for
exchange of views."
(Source: LAG website )
|
| |
|
|
Lowest dose of a chemical inducing a specified effect in a specified fraction of exposed individuals.
|
| |
|
|
The lowest tested dose of a substance that has been reported to cause harmful (adverse) health effects on people or animals.
|
| |
|
Lowest concentration or amount of a substance (dose), found by experiment or observation, which causes an adverse effect on morphology, functional capacity, growth, development, or life span of a target organism distinguishable from normal (control) organisms of the same species and strain under defined conditions of exposure.
|
| |
|
Lowest concentration of a material used in an aquatic toxicity test that has a statistically significant ad-
verse effect on the exposed population of test organisms compared with controls.
Note: When derived from a life cycle or partial life cycle test, it is numerically the same as the
upper limit of the maximum acceptable toxicant concentration (MATC). Also called
lowest-observed-adverse-effect level (LOAEL).
|
| |
|
Lowest concentration or amount of a substance (dose), found by experiment or observation, that causes any alteration in morphology, functional capacity, growth, development, or life span of target organisms distinguishable from normal (control) organisms of the same species and strain under the same defined conditions of exposure.
|
| |
|
|
Dehydration process, also known as freeze drying, in which water is removed from food and other products by sublimation in a vacuum chamber.
|
| |
|
Laboratory column of selected representative soil or a protected monolith of undisturbed field soil with
facilities for sampling and monitoring the movement of water and chemicals.
|
| |
|