|
Glossary
EVISA is providing a list of terms used in the area of speciation and fractionation analysis. Since speciation analysis is a field of analytical chemistry that is specified by a pronounced interdisciplinary cooperation between different sciences such as biochemistry, medicine, biology, environmental sciences, nutritional sciences and material sciences its terminology is a complex mixture of terms used in all these.
You may search for a term or browse the glossary alphabetically.
(In case that you cannot find the term you may consult more special glossaries or handbooks about nomenclature. For more details please consult EVISA's Link pages related to terminology,
|
Very large molecules (proteins, carbohydrates, nucleic acids), often formed by two or more identical molecules in a chain configurations (polymers).
|
| |
|
|
Imprecise term referring to a nutrient required for survival, or present in biological fluids or compartments at a level easily measured by existing analytical techniques.
|
| |
|
|
A solution of magnesium hydroxide with antacid and laxative properties.
Milk of magnesium exerts its antacid activity in low doses such that
all hydroxide ions that enter the stomach are used to neutralize
stomach acid. This agent exerts its laxative effect in higher doses so
that hydroxide ions are able to move from the stomach to the intestines
where they attract and retain water, thereby increasing intestinal
movement (peristalsis) and inducing the urge to defecate.
|
| |
|
|
The oxide salt of magnesium with antacid, laxative and vascular smooth
muscle relaxant activities. Magnesium combines with water to form
magnesium hydroxide which reacts chemically to neutralize or buffer
existing quantities of stomach acid; stomach-content and
intra-esophageal pH rise, resulting in a decrease in pepsin activity.
This agent's laxative effect is the result, in part, of osmotically
mediated water retention, which subsequently stimulates peristalsis. In
addition, magnesium ions may behave as calcium antagonists in vascular
smooth muscle.
|
| |
|
|
The magnesium salt of valproic acid (2-propylpentanoic acid) with
antiepileptic and potential antineoplastic activities. Magnesium
valproate dissociates in the gastrointestinal tract and is absorbed
into the circulation as magnesium ions and valproic acid ions; valproic
acid may inhibit histone deacetylases, inducing tumor cell
differentiation, apoptosis, and growth arrest. In addition, valproic
acid exerts an antiepileptic effect, likely by inhibiting enzymes that
catabolize the inhibitory neurotransmitter gamma-aminobutyric acid
(GABA) catabolism and so increasing concentrations of GABA in the
central nervous system (CNS). The presence of the magnesium in this
agent may contribute to its anticonvulsant activity and sedative
properties.
|
| |
|
A magnetic analyzer creates a magnetic field perpendicular to the ion path and, in conjunction with entrance and exit slits along the flight path, selects and focuses ions of a selected momentum (and, nominally, then, with the same mass-to-charge ratio) through to the detector. A magnetic analyzer is also called a magnetic sector. An instrument that includes magnetic or electric analyzers is called a sector mass spectrometer.
  
|
| |
|
|
Matrix assisted laser desorption ionization mass spectrometry (MALDI) is a mass spectrometric technique that is used for the analysis of large
biomolecules. Analyte molecules are embedded in an excess matrix of
small organic molecules that show a high resonant absorption at the
laser wavelength used. The matrix absorbs the laser energy, thus
inducing a soft disintegration of the sample-matrix mixture into free
(gas phase) matrix and analyte molecules and molecular ions. In general,
only molecular ions of the analyte molecules are produced and almost no
fragmentation occurs. This makes the method well suited for molecular
weight determinations and mixture analysis.
|
| |
|
Mancozeb is a General Use Pesticide (GUP) used to protect many fruit, vegetable, nut and field
crops against a wide spectrum of fungal diseases, including
potato blight, leaf spot, scab (on apples and pears), and rust
(on roses). It is also used for seed treatment of cotton,
potatoes, corn, safflower, sorghum, peanuts, tomatoes, flax, and
cereal grains. Mancozeb is available as dusts, liquids, water
dispersible granules, as wettable powders, and as ready-to-use
formulations. It may be commonly found in combination with zineb
and maneb.
Mancozeb is a practically nontoxic ethylene bisdithiocarbamate in
EPA toxicity class IV - practically nontoxic.
Chemical name: manganese
ethylenebis(dithiocarbamate)
Trade names: Dithane, Dithane-Ultra, Fore, Green-Daisen M,
Karamate, Mancofol, Mancozeb, Mancozin, Manzate 200, Manzeb,
Manzin Nemispor, Nemispot, Policar, Riozeb, and Zimaneb.
|
| |
|
|
Manganese is a chemical element that has the symbol Mn and atomic number 25. It is found as the free element in nature (often in combination with iron), and in many minerals. The free element is a metal with important industrial metal alloy uses. Manganese ions are variously colored, and are used industrially as pigments and as oxidants. Manganese (II) ions function as cofactors for a number of enzymes and the element is thus an essential trace mineral for all known living organisms.
|
| |
|
|
A substance preventing the interfering reaction of one ormore foreign substances in a determination by conversion into solublecomplexes, different oxidation states, or other unreactive forms.
|
| |
|
A figure which defines the range of uncertainty over which the measured m/z value has been
measured. This is the primary determinant of the possible elemental compositions which fit this
figure. See accurate mass
|
| |
|
|
Designates the kinetic energy spectrum of ions with a specific mass number and corresponds to the spectra of all product ions generated from a specific precursor ion. Using a reverse double-focusing mass spectrometer with a magnetic field --> electric field sequence, the precursor ions pass through a fixed magnetic field and are then scanned in the electric field to produce the spectrum.
|
| |
|
|
Part of the mass spectrometer that separates the ions according to their m/z ratio. Once ions have been formed and introduced into the vacuum, they are subjected to electrical (DC and/or RF) or magnetic fields. Their motion under these conditions is a function of many parameters but all include the mass-to-charge ratio. Ions can be ejected from the analyser one m/z at a time (sequential mass analyzer) or can be detected and measured simultaneously.
|
| |
|
|
Mass bias is the deviation of measured isotope ratios by a mass spectrometer from the "true value", due to mass discrimination that occurs in all mass spectrometers. In particular, several factors have been found to affect bias in ICP-MS, such as detector "dead time", the electrostatic ion focussing lens and the "space charge" effect occuring at the interface region. Compared with conventional ICP-MS, the hexapole ICP-MS hass high mass bias at low mass region, but lower mass bias at higher mass.
|
| |
|
The term mass defect has an “official” meaning that is quite different from one of its meanings in mass spectrometry. Officially, the mass defect is the difference in the mass of an atom and the sum of the masses of all of the particles (electrons, protons, and neutrons) of which it is composed. This mass defect occurs because matter is converted into energy according to the Einstein equation; this energy binds the nucleus together and overcomes the mutual repulsion between protons. In mass spectrometry, the mass defect is the term also used for the difference (whether positive or negative) between the exact mass of an ion, and the nearest integer mass.
  
|
| |
|
|
Record obtained by detecting ion intensities in real time for specific mass numbers using selective ion detection with GCMS or other methods.
|
| |
|
|
Mass-independent (isotope) fractionation (MIF) refers to any chemical or physical process that acts to separate isotopes, where the amount of separation does not scale in proportion with the difference in the masses of the isotopes. Most isotopic fractionations (including typical kinetic fractionations and equilibrium fractionations) are caused by the effects of the mass of an isotope on atomic or molecular velocities, diffusivities or bond strengths. Mass-independent fractionation processes are less common, occurring mainly in photochemical and spin-forbidden reactions. Observation of mass-independently fractionated materials can therefore be used to trace these types of reactions in nature and in laboratory experiments.
|
| |
|
Massive-cluster impact ionization (MCI) is a method for generating ions by bombarding a liquid matrix sample solution spread on a metallic target plate with multiply charged cluster ions of glycerin accelerated to 10 ~ 20kV. The clusters are proto-nated 20 ~ 300 times, have masses of 107 or greater, and have diameters on the sub-micro level (~0.01µm). The measurement method is the same as FAB. The multiply-charged cluster ions are gen-erated by electrohydrodynamic ionization.
|
| |
|
The Mathieu stability diagram is a graphical representation for reduced variables that incorporate the values of dc and ac voltages applied either to the four rods of a quadrupole mass filter or to the electrodes of an ion trap. The stability diagram illustrates areas of ion stability and ion instability and designates scan lines for the changes in those voltages so that the device can serve as an ion mass-to-charge ratio analyzer.
  
|
| |
|
|
in analysis: refers to the analytical sample, considered as an assemblage of constituents, with all their individual properties. The combined effect that the various constituents of the matrix may exert on the measure of the analysis of the element is referredto as the matrix effect.
|
| |
|
|
The combined effect (interference) of all components of the sample other than analyte on the measurement of quantity.
|
| |
|
|
are normally real world materials, which contain the analytes in the natural form and natural environment. These materials should resemble as much as possible the matrix of the sample to be analysed. When introduced in the beginning of the analysis, these materials can used to asses the quality of the analytical process process in all its steps.
|
| |
|
|
Matrix-assisted plasma desorption (MAPD) is a mass spectrometric technique using plasma desorption with the sample dissolved in a liquid or solid matrix.
|
| |
|
External calibration using calibration standards prepared in matrix blank extract, which compensate for specific matrix effects.
|
| |
|
|
The Mattauch-Herzog geometry is an instrumental design used in double-focusing mass spectrometers
when the electric field and magnetic field are arranged at deflection
angles of π/(4√2) and π /2 radians respectively.
|
| |
|
|
Designates rearrangement of hydrogen atoms, through a 6-membered ring, to a specific heteroatom. The term is also often used to describe the fragmentation that occurs simultaneously at the γ bond position of the heteroatom.
|
| |
|
|
The arithmetic average of a set of values. A
measure of central tendency of the distribution of a set of replicate
results. Often abbreviated by an x with a bar over it.
|
| |
|
Average time a substance remains in an animal body or an organ after rapid intravenous injection.
Note 1: Like clearance, its value is independent of dose in most cases.
Note 2: After an intravenous bolus
tr= Am/A
where tr is the MRT, A is the area under the plasma concentration-time curve, and Am is
the area under the moment curve.
Note 3: For a drug with one-compartment distribution characteristics, MRT equals the recipro-
cal of the elimination rate constant.
|
| |
|
Meglumine antimoniate, commercialized as Glucantime, is the most common treatment for leishmaniasis over the last 70 years. It belongs to a group of compounds known as the pentavalent antimonials. It is administered by intramuscular injection. The active ingredient in treating leishmaniasis is believed to be antimonite Sb(III) after in vivo reduction of antimoniate Sb(V).
|
| |
|
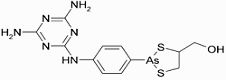 Melarsoprol (INN) is a medicinal drug used in the treatment of trypanosomiasis, such as Chagas disease and West African sleeping sickness, the former caused by Trypanosoma cruzi and the latter by Trypanosoma brucei gambiense. It is also sold under the trade names “Mel B” and “Melarsen Oxide-BAL.” Melarsoprol (INN) is a medicinal drug used in the treatment of trypanosomiasis, such as Chagas disease and West African sleeping sickness, the former caused by Trypanosoma cruzi and the latter by Trypanosoma brucei gambiense. It is also sold under the trade names “Mel B” and “Melarsen Oxide-BAL.”
Being a toxic organic compound of arsenic, melarsoprol is a highly dangerous treatment which is only administered by injection under the supervision of a physician, as it can produce similar effects as arsenic poisoning. Negative side effects caused by Mlarsoprol include convulsions, fever, loss of consciousness, rashes, bloody stools, nausea, and vomiting. It is fatal in and of itself in around 8% of cases.
IUPAC name: (2-(4-(4,6-diamino-1,3,5-triazin-2-ylamino)phenyl)-1,3,2-dithiarsolan-4-yl)methanol
Formula: C12H15AsN6OS2
Mol. mass: 398.341 g/mol
CAS number: 494-79-1
|
| |
|
In biology a thin, pliable layer of tissue covering surfaces or separating or connecting regions, structures, or organs of an animal or a plant.
In chemistry a membrane is a thin sheet of natural or synthetic material that can be penetrated, especially by liquids or gases.
In environmental applications of nanotechnology a membrane can be used as a filter.
|
| |
|
|
A progressive brain disease associated with copper deficiency caused by
a sex-linked inherited disorder, resulting in defective
gastrointestinal
absorption of copper early in
infancy.
|
| |
|
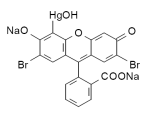 Merbromin (marketed as Mercurochrome, Merbromine, Sodium mercurescein, Asceptichrome, Supercrome and Cinfacromin) is an organomercuric disodium salt compound and a fluorescein. Merbromin's best-known use is as a topical antiseptic for minor cuts and scrapes; however, along with Merthiolate, it has been ruled ineffective by the FDA, and is no longer approved in the US but still available in many other countries. Merbromin (marketed as Mercurochrome, Merbromine, Sodium mercurescein, Asceptichrome, Supercrome and Cinfacromin) is an organomercuric disodium salt compound and a fluorescein. Merbromin's best-known use is as a topical antiseptic for minor cuts and scrapes; however, along with Merthiolate, it has been ruled ineffective by the FDA, and is no longer approved in the US but still available in many other countries.
Mol. wt.: 750.70; freely soluble in water, giving a carmine-red solution.
|
| |
|
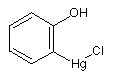 o-(Chloromercuri)phenol; o-hydroxyphenylmercuric chloride o-(Chloromercuri)phenol; o-hydroxyphenylmercuric chloride
Poisonous compound used as a disinfectant.
Mol. wt.: 329.18; mp 150-152°C; Slightly soluble in cold water, moderately soluble in boiling water
The Food and Drug Administration has reclassified Mercufenol chloride (to category III, insufficient data to classify
in Category I or II) with the effect
that this product is no longer approved for over-the-counter sale.
|
| |
|
Mercury, also called quicksilver, is a chemical element in the periodic table that has the symbol Hg (Latinized Greek: hydrargyrum, meaning watery or liquid silver) and atomic number 80. A heavy, silvery transition metal, mercury is one of five elements that are liquid at or near room temperature and pressure. (The others are the metals caesium, francium, and gallium, and the non-metal bromine.)
Mercury is used in thermometers, barometers and other scientific apparatus, though concerns about the element's toxicity have led to mercury thermometers being largely phased out in clinical environments in favour of alcohol-filled, digital or thermistor-based instruments. It remains in use in a number of other ways in scientific and scientific research applications, and in dental amalgam. Mercury is mostly obtained by reduction from the mineral, cinnabar.
Mercury occurs in deposits throughout the world and it is relatively harmless in an insoluble form, such as mercuric sulfide, but it is poisonous in soluble forms such as mercuric chloride or methylmercury.
|
| |
|
Enclosed and essentially self-sufficient (but not necessarily isolated) experimental environment or
ecosystem that is on a larger scale than a laboratory microcosm.
Note: A mesocosm is normally used outdoors or, in some manner, incorporated intimately
with the ecosystem that it is designed to reflect.
|
| |
|
|
The entire set of enzyme-catalyzed transformations of organic nutrient molecules (to sustain life) in living cells. Conversion of food and water into nutrients that can be used by the body’s cells, and the use of those nutrients by those cells (to sustain life, growth, etc.).
|
| |
|
|
Intermediate or product resulting from metabolism.
|
| |
|
|
The set of metabolites produced as a result of reactions catalysed by certain proteins (enzymes)
|
| |
|
|
Whilst genomics is concerned with the measurement of all the genes is a system and proteomics all the proteins, the science of metabolomics is concerned with the attempted measurement of all the metabolites in a given cell or system. Metabolites are extremely important as they represent the endpoints in biochemical pathways and contribute to the resultant phenotype. Measurement of these metabolites is a complex science requiring the use of many techniques and the integration of the subsequent data sets.
|
| |
|
The quantitative measurement of the dynamic multiparametric metabolic response of living systems
to pathophysiological stimuli or genetic modification.
see also: metabolomics
|
| |
|
|
Water quality parameter determining the concentration of heavy metals that can be discharged to a waterway before free metal ion appear because of complexation by (natural) complexing agents such as humic substances.
|
| |
|
MeCAT is a proteomics quantification technology using metal coded tags (lanthanides) for marking biomolecules without the
necessity of isotopic labeling. After that they are analysed with
state-of-the-art mass spectrometric methods including ICP-MS with its
extraordinary advantages in quantification.
ICP-MS (inductively coupled plasma mass spectrometry) allows an ultra sensitive (absolute) quantification of the metals (lanthanides) tagged to proteins on protein level. Thus the protein amount can be accurately determinated down to low attomol range which is at least 2 to 3 orders of magnitude more sensitive than other mass spec (peptide based) techniques.
|
| |
|
|
A species formed between a metal ion and an anion or neutral species (i.e. ligand). The ligand possesses atoms with nonbonding electron pairs that are donated to form coordinate covalent bonds with the metal cation.
|
| |
|
|
A technique in which antigen-antibody recognition is used, with attachment of a metal ion or metal complex to the antibody. The specific absorption or (radioactive) emission of the metal is then used as a probe for the location of the recognition sites.
|
| |
|
|
In chemistry, and in particular, in organometallic chemistry, a metallocene is a compound consisting of an aromatic organic ligand bound to a metal.
A key feature of metallocenes is planar aromatic organic ligands in which multiple of the carbon atoms in the ring form metal-carbon bonds that are equivalent.
The prototypical metallocene is ferrocene, consisting of two cyclopentadienyl rings bound on opposite sides of a central iron atom, forming an organometallic sandwich compound. |
| |
|
|
Metallochaperones are soluble proteins involved in metal transport and regulation in vivo. Copper metallochaperones belong to a structural family of metal binding domains displaying a ferredoxin-like fold (betaalphabetabetaalphabeta) and a consensus metal-binding motif MXCXXC. The metal-binding selectivities for this class of proteins are poorly documented so far.
|
| |
|
|
Term used to define a pharmaceutical, having a activity center containing a metal. Platinum (cisplatin, carboplatin) and gold (Auranofin) compounds are well-known in cancer therapy whereas some other gold compounds (aurithiomalate, aurothioglucose) are important antiarthritic drugs. A wide range of Tc compounds (e.g. Tc-labelled antibodies, Tc-mercaptoacetyl glycine complex) are used for diagnostic imaging of renal, cardiac and cerebral functions and of various forms of cancer. Gadolinium (III) polyaminopolycarboxylic crown complexes are widely used as contrast agents in magnetic resonance imaging.
|
| |
|
|
An enzyme that, in the active state, contains one or more metal ions which are essential for its biological function.
|
| |
|
|
Metallic compound being capable of binding to cellular oestrogen receptors and then mimicking the actions of physiological oestrogens. There is evidence that even inorganic ions of aluminium, antimony, arsenite, barium, cadmium, chromium (Cr(II)), cobalt, copper, lead, mercury, nickel, selenite, tin and vanadate have such potential.
|
| |
|
|
Together with the metals and nonmetals, the metalloids (in Greek metallon = metal and eidos = sort - also called semimetals) form one of the three categories of chemical elements as classified by ionization and bonding properties. They have properties intermediate between those of metals and nonmetals. There is no unique way of distinguishing a metalloid from a true metal but the most common is that metalloids are usually semiconductors rather than conductors.
The known metalloids (and their atomic symbols) are: Boron (B), Silicon (Si), Germanium (Ge), Arsenic (As), Antimony (Sb), Tellurium (Te) and Polonium (Po)
|
| |
|
The entirety of metal and metalloid species within a cell or tissue type. It encompasses, among others, the inorganic species (ionome) and protein complexes (metalloproteome).
|
| |
|
|
Integrated research field related to biometals and in symbiosis with genomics and proteomics. In metallomics, metalloproteins, metalloenzymes and other metal- containing biomolecules are defined as ‘‘metallomes’’, in a similar manner to genomes in genomics as well as proteomes in proteomics. In the study of metallomics, elucidation of the physiological roles and functions of biomolecules binding with metallic ions in the biological systems should be the most important research target. Then, metallomics may be called, in another words, ‘‘metal-assisted function biochemistry’’.
|
| |
|
|
Metalloproteins are proteins containing a metal ion cofactor that is required for the protein's biological activity. It is estimated that approximately half of all proteins contain a metal. Metalloproteins have many different functions in cells, such as enzymes, transport and storage proteins, and signal transduction proteins. In metalloproteins, metal ions are usually coordinated by nitrogen, oxygen or sulfur centres belonging to amino acid residues of the protein. In addition to donor groups that are provided by amino acid residues, a large number of organic cofactors function as ligands.
|
| |
|
|
The entirety of metal complexes with proteins in a sample. Note that this term had originally been used in a narrower sense and concerned the proteins with enzymatic fuctions only
|
| |
|
|
- a cysteine-rich, heat-stable, low-molecular weight protein which complexes metals such as cadmium, copper, mercury, and zinc. Functions attributed to MT include detoxification, storage, and regulation of these metals.
|
| |
|
Poorly characterized, cysteine-rich metal-binding proteins or proteins not conforming precisely to the
classic properties of metallothioneins.
|
| |
|
A metastable ion is a precursor ion that dissociates into a fragment ion and neutral species after leaving the ion source (that is, after acceleration) but before reaching the detector. The dissociation is most readily observed when it takes place in one of the field-free regions of a sector mass spectrometer.
  
|
| |
|
|
A metastable peak is a peak on the mass spectrum generated by ions created from decay of metastable ions in flight.
|
| |
|
|
An essential amino acid; furnishes (to organism) both labile methyl groups and sulfur necessary for normal metabolism.
|
| |
|
|
The overall, systematic procedure required to undertaken an analysis. This includes all stages of the analysis, and not just the (instrumental) end determination.
|
| |
|
The minimum concentration of a substance that can be measured and reported with 99% confidence that the analyte concentration is greater than zero. It is determined by analysis of a sample in a given matrix containing the analyte.
(see also: instrumental detection limit)
|
| |
|
|
The process of testing a measurement
procedure to assess its performance and to validate that performance is
acceptable. The magnitudes of the analytical errors are experimentally
determined and their acceptability for the application of the method is
judged versus defined requirements for quality in the form of maximum
allowable errors.
|
| |
|
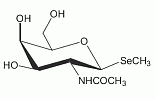 This selenosugar has been identified as the main selenium metabolite occuring in human background urine and in urine after ingestion of sodium selenite, L-selenomethionine or DL-selenomethionine. This selenosugar has been identified as the main selenium metabolite occuring in human background urine and in urine after ingestion of sodium selenite, L-selenomethionine or DL-selenomethionine.
|
| |
|
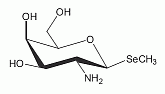 The deacylate analog of the selenosugar methyl-2-acetamido-2-deoxy-1-seleno-ß-D-galactopyranoside has been identified as a minor Se-metabolite in human urine. The deacylate analog of the selenosugar methyl-2-acetamido-2-deoxy-1-seleno-ß-D-galactopyranoside has been identified as a minor Se-metabolite in human urine.
|
| |
|
(CH3AsS)x : A colorless compound whose flakes melt at 110°C;
insoluble in water; used as a fungicide in treating cotton seeds. Also
known as thioarsorosomethane (Rhizoctol, Asozin, MAS, Monkil or Wrabasulf : CAS 2533-82-6, MW 122.023)
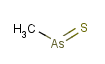
|
| |
|
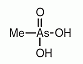 Methylarsonic acid (MAV) is an organoarsenic compound found as a minor metabolite in urine of humans and animals after ingestion of inorganic arsenic compounds. Methylarsonic acid (MAV) is an organoarsenic compound found as a minor metabolite in urine of humans and animals after ingestion of inorganic arsenic compounds.
|
| |
|
Addition of a methyl group to a molecule or atom, either by a microbial activity (biomethylation) or by purely chemical reaction (abiotic methylation).
|
| |
|
|
Methylcyclopentadienylmanganese tricarbonyl (MMT) has been used as a partial substitute for alkyllead compounds in its role as an antiknock compounds to improve the petrol octane rating.
Formula: C9H7MnO3
Molecular Weight: 218.09
CAS Registry Number: 12108-13-3
|
| |
|
|
Alkylmercury compounds having methyl groups. Includes both mono- and dimethylmercury. In fact,
methylmercury is not a compound in itself but a cation,
CH3Hg+, which forms one part of methylmercury compounds;
usually methylmercury salts.
Dimethylmercury is one methylmercury
compound that is not a salt. The methylmercury cation is normally associated
with either a simple anion, like chloride (Cl-), or a large
molecule (e.g. a
protein) with negative and positive
charges. The methylmercury cation is the most
toxic form of mercury, able to inhibit fetal
brain development, which results in the behavioural changes and reduced
cognitive and motor ability.
|
| |
|
Methylselenocysteine, sometimes called Se-methylselenocysteine. The methylated form of SeCys. Produced naturally by brassicas, alliums and Se hyperaccumulators when grown in the presence of Se (as
selenate, selenite or certain organoselenium compounds), but at very low amounts in other crops.
|
| |
|
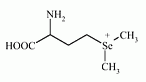 Methylselenomethione has been reported as a metabolite of the Se metabolism in plants and animals. Molecular formula: C6H14NO2Se+
IUPAC Name: (3-amino-3-carboxy-propyl)-dimethyl-selenonium
Molecular Weight: 211.141 g/mol
|
| |
|
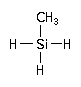 Methylsilane is used in the epitaxial Chemical Vapor Deposition (CVD) of germanium doped silicon carbide (Si-Ge-C) layers.
CAS Number : 992-94-9
|
| |
|
|
A chemical species containing pentavalent arsenic, sulfide, and methyl groups, as examples: (CH3)AsO2S2−, (CH3)AsOS22−, (CH3)2AsOS−, and (CH3)2AsS2− (compare with thioarsenic, thioarsenate, and thioarsenite).
|
| |
|
|
An enzyme that catalyzes the transfer of a methyl group from one molecule to another.
|
| |
|
|
the science of measurement and its application
|
| |
|
|
The main separation mechanism is based on solute partitioning between the micellar phase and the solution phase. The technique provides a way to resolve neutral molecules as well as charged molecules by CE. Micelles form in solution when a surfactant is added to water in concentration above its critical micelle concentration (CMC). Even though these anionic micelles are attracted toward the anode, in an uncoated fused silica capillary they still migrate toward the cathode because of electroosmotic flow. However, the micelles move toward the cathode at a slower rate than the bulk of the liquid because of their attraction towards the anode. Neutral molecules partition in and out of the micelles based on the hydrophobicity of each analyte. Consequently the micelles of MEKC are often referred to as pseudo (or moving) stationary phase. A very hydrophilic neutral molecule, eg methanol, will spend almost no time inside the micelle and will therefore migrate essentially at the same rate as the bulk flow and elute earlier. On the other hand, a very hydrophobic neutral molecule, eg Sudan III, will spend nearly all the time inside the micelles and will therefore elute later, together with the micelles. All other solutes with intermediate hydrophobicity will migrate within this migration window. MEKC can be used with ionic substances as well as neutral compounds. A combination of charge/mass ratios, hydrophobicity and charge interactions at the surface of the micelles combine to affect the separation of the analytes.
|
| |
|
|
Separation mode in capillary electrophoresis, separating according to the ability of apolar analytes to enter the (apolar) core of surface charged micelles
|
| |
|
- an aggregate of surfactant molecules that forms when the surfactant is present at a concentration at or above its CMC. Micelles form in order to make the surfactant more stable in solution. In water, micelles form such that the ouside of the micelle is hydrophilic and the inside is hydrophobic. This arrangement allows the surfactant to remain in solution at higher concentrations
than would be otherwise possible.
|
| |
|
|
Refers collectively to techniques in which a column of smaller than conventional inner diameter is used for separation. The term micro-HPLC most often is used for HPLC in columns with inner diameters smaller than 0.5 mm; micro-HPLC is used in high-sensitivity analysis when the sample amount is limited and with certain ionization techniques in LC–MS in which the volume of solvent flowing into the ionization source must be minimized.
|
| |
|
|
Particle-induced x-ray emission spectroscopy in which the particle beam is highly collimated and focused allowing position sensitive analysis
|
| |
|
A thin plate that contains a closely spaced array of channels that each act as a continuous dynode
particle multiplier. A charged particle, fast neutral particle, or photon striking the plate causes a
cascade of secondary electrons that ultimately exits the opposite side of the plate.
|
| |
|
Artificial multi-species test system that simulates major characteristics of the natural environment for
the purposes of ecotoxicological effects and risk assessment; such systems are normally terrestrial or
aquatic and may contain plants, animals (vertebrates and invertebrates), and microorganisms.
Note: The terms mesocosm and macrocosm are used to refer to larger and more complex sys-
tems than microcosms, but the distinction is often not clearly defined.
|
| |
|
|
Electrode with at least one dimension small enough for nonlinear diffusion to occur at the electrode surface. The critical dimension of these electrodes falls in the range of 0.1 to 50 µm.
|
| |
|
|
a clear thermodynamically stable dispersions of two immiscible liquids containing appropriate amounts of surfactants and cosurfactants.
|
| |
|
|
A multidisciplinary field comprising physics, chemistry, engineering and biotechnology that studies the behavior of fluids at volumes thousands of times smaller than a common droplet. Microfluidic components form the basis of so-called “lab-on-a-chip” devices that can process microliter and nanoliter volumes and conduct highly sensitive analytical measurements. The fabrications techniques used to construct microfluidic devices are relatively inexpensive and are amenable both to highly elaborate, multiplexed devices and also to mass production. In a manner similar to that for microelectronics, microfluidic technologies enable the fabrication of highly integrated devices for performing several different functions on the same substrate chip. Microfluidics is a critical component in gene chip and protein chip development efforts.
|
| |
|
|
A method of digesting an organic matrix to liberate metal content by using an acid at elevated temperature (and pressure) based on microwave radiation. Can be carried out in either open or sealed vessels.
|
| |
|
|
Microwave-assisted extraction (MAE) is an efficient sample preparation technique that has been demonstrated for different solid matrices (e.g. soils, sediments and biological tissues) and constitutes a valuable tool for the rapid treatment of solid samples in organometallic speciation analysis. A careful optimization of the conditions of the microwave extraction procedure is required, in terms of stability of the target compounds in a microwave field, prior to speciation analysis. Essential parameters for the optimization are the extraction medium, power applied and the exposure time. MAE is considerably faster than other sample preparation and extraction procedures: a typical sample treatment may take only 3 min.
An important drawback of microwave-assisted acid extraction or leaching is that analytes in the polar leachate have to be derivatized and transferred into a non-polar solvent to produce a solution suitable for GC analysis and this increasses the number of steps in the analytical procedure. This obstacle can be overcome by combining leaching, derivatization and liquid-liquid solvent extraction to produce directly a solution of the analytes for GC by the so called microwave-assisted derivatization solvent extraction (MADSE) technique.
|
| |
|
Microwave-induced plasma consists of a quartz tube surrounded by a
microwave wave guide or cavity. Microwaves produced from a magnetron (a
microwave generator) fill the wave guide or cavity and cause the
electrons in the plasma support gas to oscillate. The oscillating
electrons collide with other atoms in the flowing gas to create and
maintain a high-temperature plasma. Typically 2.45 GHz, the same frequency as used in microwave ovens (l ~ 1.22 cm) is used for plasma generation. The MIP is typically used as a source for atomic emission spectrometry (MIP-AES) or mass Spectrometry (MIP-MS).
|
| |
|
|
Taking a sample of a flowing liquid, such as urine, avoiding initial and terminal flow periods, which are likely to be unrepresentative.
|
| |
|
|
- the movement of ionic solutes between opposite electrodes during electrophoresis.
|
| |
|
|
- the time taken for an ionic solute to move the length of the capillary to the detector.
|
| |
|
|
- the speed with which ionic solutes move through a capillary during electrophoresis.
|
| |
|
Membrane inlet mass spectrometry (MIMS)
A membrane inlet system consists of a semipermeable membrane that permits passage of gas-phase volatile sample molecules directly into the mass spectrometer ion source, which is usually operated as an electron ionization or chemical ionization source.
|
| |
|
|
a computer program for the calculation of chemical equilibrium composition of aqueous systems.
|
| |
|
An ATSDR estimate of daily human exposure to a hazardous substance at
or below which that substance is unlikely to pose a measurable risk of
harmful (adverse), noncancerous effects. MRLs are calculated for a route
of exposure (inhalation or oral) over a specified time period (acute,
intermediate, or chronic). MRLs should not be used as predictors of harmful
(adverse) health effects [see reference dose].
Source: ATSDR Glossary of Terms
|
| |
|
|
geochemical speciation model based on equilibrium thermodynamics which can calculate the equilibrium composition of dilute aqueous systems amongst soluble, solid, adsorbed and gas phases. Calculations can be performed to take into account varied environmental conditions such as pH, ionic strength, temperature and redox conditions. The program also includes an extensive database which includes thermody<namic data for the soluble complex, mineral solubilities, gas solubilities and redox couples. In addition more recent updates contain a database of metal surface complexation constants and complexation constants for metal complexation with dissolved organic matter.
|
| |
|
The ability of a chemical element or a pollutant to move into and
through the environment (e.g., the mobilization of an element from a
water column to sediment).
|
| |
|
A molecular ion is formed by the removal (positive ions) or addition (negative ions) of one or more electrons from a molecule M to form M+ or M-. The mass of the molecular ion corresponds to the nominal or monoisotopic mass of the molecule, with the mass of the electron added or lost usually consequential. Of course, the mass of such a molecular ion reflects the isotopic composition of the ion, rather than the average molecular mass of the molecule. Thus the molecular ion mass is the sum of the relative masses of the most abundant naturally occurring isotopes of the various atoms that make up the molecule.
  
|
| |
|
- a gray metal or black powder. Used as an alloying agent in steels and cast iron and nonferrous alloys. Molybdenum is also a core ingredient of important chemicals such as pigments for printing inks, paints, ceramics and hair dye, lubricants, photochemicals and catalysts. Molybdenum is an essential element both for plants and animals. It is used in agriculture to prevent molybdenum deficiency in crops.
Hazard: Flammable in form of dust or powder. Acute exposures may include severe gastrointestinal irritation with diarrhea, coma, and death from heart failure.
|
| |
|
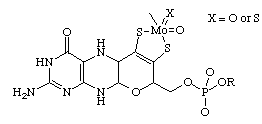 The molybdenum complex of the molybdopterin prosthetic group (ligand). In the molybdenum cofactor the minimal coordination of the Mo atom is thought to be provided by the chelating dithiolenato group of the molybdopterin and either two oxo or one oxo and one sulfido ligands
|
| |
|
|
An optical device that transmits a selectable narrow band of wavelengths of light chosen from a wider range of wavelengths available at the input, meaning that a single color or wavelength is being selected at a time.
|
| |
|
|
The monodisperse aerosol generating interface for chromatography (MAGIC) is a
variant of the particle beam interface that incorporates a monodisperse
aerosol generator.
|
| |
|
The monoisotopic ion mass is defined as the mass of an ion for a given empirical formula calculated using the exact mass of the most abundant isotope of each element; for example, C = 12.000000 Da (exactly), H = 1.007825 Da, O = 15.994915 Da.
  
|
| |
|
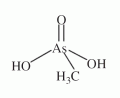 An organic pentavalent arsenical with the composition of (CH3)AsO(OH)2, abbreviated MMA(V), see Figure (compare with dimethylarsinous acid, dimethylarsinic acid, and monomethylarsonous acid). An organic pentavalent arsenical with the composition of (CH3)AsO(OH)2, abbreviated MMA(V), see Figure (compare with dimethylarsinous acid, dimethylarsinic acid, and monomethylarsonous acid).
|
| |
|
|
Organic form of mercury with one methyl group attached to a mercury atom (CH3Hg)+ - highly toxic and readily accumulated by living organisms
|
| |
|
|
The organoselenium compound monomethylselenol (MMSe) has been identified as a Se-metabolite in the urine of rats.
|
| |
|
Analysis of a sequence of events using random numbers to generate possible outcomes in an iterative
process.
|
| |
|
|
For the morphological characterisation (shape, size, roughness) of individual atmospheric particles, especially of asbestos, mineral fibres and quartz, a scanning electron microscope (SEM), transmission electron microscope (TEM), and a light microscope can be used, as well as quantitative determination of crystalline silica in respirable-size dust samples by infrared spectrophotometry.
|
| |
|
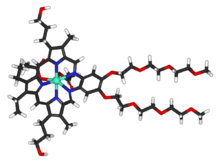 Motexafin gadolinium (MGd) is a type of metalloporphyrin complex also called gadolinium texaphyrin with a novel
mechanism of action; it accumulates selectively in cancer cells due to
their increased rates of metabolism. Once inside the cell, MGd induces
apoptosis (programmed cell death) by disrupting redox-dependent
pathways. MGd inhibits the enzyme thioredoxin reductase, which is a
tumor growth promoter. This mechanism provides the opportunity to use
MGd in a wide range of cancers. MGd is paramagnetic, and therefore is
detectable by magnetic resonance imaging (MRI), allowing the
visualization of the drug in tumors. Motexafin gadolinium (MGd) is a type of metalloporphyrin complex also called gadolinium texaphyrin with a novel
mechanism of action; it accumulates selectively in cancer cells due to
their increased rates of metabolism. Once inside the cell, MGd induces
apoptosis (programmed cell death) by disrupting redox-dependent
pathways. MGd inhibits the enzyme thioredoxin reductase, which is a
tumor growth promoter. This mechanism provides the opportunity to use
MGd in a wide range of cancers. MGd is paramagnetic, and therefore is
detectable by magnetic resonance imaging (MRI), allowing the
visualization of the drug in tumors.
Formula: C52H72GdN5O14
Mol. mass: 1148.403 g/mol
|
| |
|
|
A pentadentate aromatic metallotexaphyrin with photosensitizing
properties. Motexafin lutetium preferentially accumulates in tumor
cells due to their increased rates of metabolism and absorbs light,
forming an extended high energy conformational state that produces high
quantum yields of singlet oxygen, resulting in local cytotoxic effects.
|
| |
|
|
The maximum concentration of residue resulting from the use of a veterinary drug (expressed in mg/kg or mg/kg on a fresh weight basis) that is acceptable in or on a food. It is based on the type and amount of residue considered to be without toxicological hazard for human health as expressed by the Acceptable Daily Intake (ADI), or on the basis of a temporary ADI that utilizes an additional safety factor. It also takes into account other relevant public health risks as well as food technological aspects and estimated food intakes. When establishing an MRL, consideration is also given to residues that occur in food of plant origin and/or the environment. Furthermore, the MRL may be reduced to be consistent with good practices in the use of veterinary drugs and to the extent that practical analytical methods are available.
|
| |
|
|
Multiple/Selected Reaction Monitoring is a special mass spectrometric detection mode in which a specific reaction species or multitude through the collision or reaction cell is monitoired, giving a clear spectrum suited for target analysis using tandem MS setups.
|
| |
|
A molecule which has more than one ligand atom available for binding
with a central metal atom. The atoms involved can be the same or
different.
see also:
- bidendate – 2 ligand atoms
- tridendate – 3 ligand atoms
- tetradendate – 4 ligand atoms
- hexdentate – 6 ligand atoms
|
| |
|
|
The use of two or more columns or chromatographic techniques for improved separation. It is useful for sample cleanup, increased resolution, increased throughput, and increased peak capacity. It can be used off-line by collecting fractions and reinjecting them onto a second column or on-line by using a switching valve. Also called coupled column chromatography, column switching, multicolumn chromatography, and boxcar chromatography.
|
| |
|
Multiple reaction monitoring (MRM) is an MS/MS experiment that uses two sequential stages of independent mass analysis. In the product ion MS/MS scan, a precursor ion is selected by mass with the first mass analyzer, and the fragment ions formed as a result of collision-induced dissociation are measured with a scan of the second mass analyzer and recorded in a mass spectrum. In an analogy to selected ion monitoring, if both mass analyzers in an MS/MS instrument are set on a specific mass, the signal represents the precursor-to-product ion transition for a specific ion pair. This experiment is called reaction monitoring. If several different precursor–product ion pairs are monitored, as is most often the case, the experiment is multiple reaction monitoring.
  
|
| |
|
The degree to which a substance can cause a change in an organisms's DNA.
|
| |
|
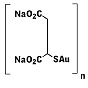 First
generation water-soluble gold(1) thiolate metallodrug used for
treatment of rheumatoid arthritis. Serious side effects led to the
development of second generation gold drugs such as auranofin. First
generation water-soluble gold(1) thiolate metallodrug used for
treatment of rheumatoid arthritis. Serious side effects led to the
development of second generation gold drugs such as auranofin.
IUPAC name : auriothiomalate
|
| |
|