|
Glossary
EVISA is providing a list of terms used in the area of speciation and fractionation analysis. Since speciation analysis is a field of analytical chemistry that is specified by a pronounced interdisciplinary cooperation between different sciences such as biochemistry, medicine, biology, environmental sciences, nutritional sciences and material sciences its terminology is a complex mixture of terms used in all these.
You may search for a term or browse the glossary alphabetically.
(In case that you cannot find the term you may consult more special glossaries or handbooks about nomenclature. For more details please consult EVISA's Link pages related to terminology,
|
|
in spectroscopy: Data value in a datastream in the absence of any analyte or interference.
|
| |
|
|
The concentration (or level) of a substance characteristic of a particular medium (e.g. soil, water, rock etc.) in an area or region arising from both natural sources and non-natural diffuse sources such as atmospheric deposition. (after definition for background concentration soil, ISO 11074-1:1996)
|
| |
|
|
The BEC is the magnitude of a signal in a blank, expressed as a concentration
|
| |
|
Levels of chemical or physical agents that are normally found in the
environment.
Two types of background levels may exist for chemical substances or physical
agents: (a) Naturally occurring levels: ambient concentrations of substances or
agents present in the environment, without human influence; (b) Anthropogenic
levels: Concentrations of substances or agents present in the environment due to
human-made, non-site sources (e.g., automobiles, industries).
(Source:
US EPA Glossary of IRIS Terms )
|
| |
|
The mass spectrum observed when no sample is intentionally introduced into the mass spectrometer or spectrograph.
Source: IUPAC
|
| |
|
|
The process of removing contaminants from a column by operation at elevated temperatures, which should not exceed a column’s maximum operating temperature (MAOT).
|
| |
|
|
The process of increasing width and concomitant diluting of the chromatographic band as it moves down the column. The peak is injected as a narrow slug and, ideally, each separated component would be eluted as a narrow slug of pure compound if not for the process of band broadening. The measure of band broadening is bandwidth (tw) or, more correctly, the number of theoretical plates (N) in the column. Sometimes called band dispersion or band spreading.
|
| |
|
|
A mechanism used in a dynamic reaction cell (DRC) to reject the by-products generated through secondary reactions utilizing the principle of mass discrimination. Achieved by optimizing the electrical fields of the reaction cell multipole (typically a quadrupole) to allow transmission of the analyte ion, while rejecting the polyatomic interfering ion.
|
| |
|
|
The base peak in a mass spectrum (within the user-selected mass range) is the ion with the highest measured abundance. The relative abundance of the base peak ion is assigned a value of 100, and the abundances of all the other ions plotted in that mass spectrum are normalized to that value. The y-axis in a mass spectrum is therefore given in terms of relative abundance.
|
| |
|
|
A leaching test that estimates how readily contaminants could mobilize out of a solid waste or other solid material if the material comes into contact with natural waters. The test involves placing a given mass of the solid sample in a container with a specific volume of a liquid leaching solution. The leaching solution may be water, salt buffers, acidic solutions, basic solutions, or organic solvents. The mixture is agitated for a specific amount of time (usually, hours to days). Afterwards, the mixture is filtered and the liquid (the leachate) is analyzed for contaminants, such as arsenic. A common batch leaching method is the US Environmental Protection Agency’s toxicity characteristic leaching procedure (TCLP), which is used to determine whether a solid or liquid waste has the toxicity characteristic of hazardousness (compare with leaching, leaching test, column leaching, sequential batch leaching, and serial batch leaching).
|
| |
|
|
consensus sequential extraction schemes on the basis of sequential solubility of metals bound to a substrate making up sediments and soils. This approach is necessary to ensure comparability of the data corresponding to operationally defined parameters.
|
| |
|
A term used to distinguish between laboratory-scale or small-scale processes, those that can be performed "on the bench" (in the lab or even on a tabletop) and larger, pilot- or production-scale processes. Benchtop equipment (a "benchtop bioreactor", for example) can fot on a table or in a confined laboratory area.
|
| |
|
|
Non-essential metal which in some form (chemical species) at an appropriate dose can improve health of defined organisms, commonly human beings.
|
| |
|
|
Ther Berner impactor is a 8-stage cascade impactor widely used in recent aerosol studies in Europe and in the USA. The flow rate is 1.9 m3h-1 at 20°C with an exhaust pressure of 150 hPa. The impactor consists of eight stages with cut-off diameters of 8/4/2/1/0.5/0.25/0.125/0.0625 µm and a prestage excluding particles > 16 µm.
|
| |
|
Serious and usually permanent lung damage resulting from chronic inhalation of beryllium. (IUPAC)
|
| |
|
a hard brittle, gray-white metal. Resistant to oxidation at ordinary temperatures. Used in computer parts, x-ray tubes, gyroscopes and rocket fuel additive.
Hazard: Highly toxic, especially by inhalation of dust. Long term exposure may cause weight loss, weakness, cough, extreme difficulty in breathing and cardiac failure.
|
| |
|
|
A systematic difference or systematic error between an observed value
and some measure of the truth. Generally used to describe the inaccuracy
of a method relative to a comparative method in a method comparison
experiment. It also has a specific meaning in the statistical t-test,
where bias equals the difference between the mean values of the two
methods being compared or the average of all the differences between the
paired sample values.
|
| |
|
A specific region (or atom) in a molecular entity that is capable of
entering into a stabilizing interaction with another molecular entity.
An example of such an interaction is that of an active site in an
enzyme with its substrate. Typical forms of interaction are by hydrogen
bonding, coordination, and ion pair formation. Two binding sites in
different molecular entities are said to be complementary if their
interaction is stabilizing.
|
| |
|
|
Bioaccessibility is a measure of the physiological solubility of a metal in the gut.
Because solubility is usually required for absorption across membranes bioaccessibility
may be a predictor of bioavailability when solubility is the major determinant of
absorption across the gut epithelium.
|
| |
|
|
Process by which some endogenous or exogenous substances, present in small quantities, increase in concentration in an organ, an organism, a food chain, or an ecosystem.
(Translated from Parent, S. Dictionnaire des sciences de l'environnement. Broquet, Québec, 1990.)
|
| |
|
|
Ratio of tissue concentration to concentration in medium, with exposure from the food web and the medium.
|
| |
|
Analytical methods that pertain to biotechnology (that is, to proteins, peptides, and other biomolecules) are more specifically referred to as bioanalytical methods.
|
| |
|
Procedure for estimating the concentration or biological activity of a substance (e.g.vitamin, hormone, plant growth factor, antibiotic,
enzyme) by measuring its effect on a living system compared to a standard system.
|
| |
|
|
extent to which a substance to which a body is exposed (by ingestion, inhalation, injection, or skin contact) reaches the systemic circulation, and the rate at which this occurs
|
| |
|
|
Substance concentration of oxygen taken up through the respiratory activity of micro-organisms growing on organic compounds present when incubated at a specified temperature (usually 20° C) for a fixed period (usually 5 days). It is regarded as a measure of that organic pollution of water which can be degraded biologically but includes the oxidation of inorganic material such as sulfide and iron(II). The empirical test used in the laboratory to determine BOD also measures the oxygen used to oxidize reduced forms of Nitrogen unless their oxidation is prevented by an inhibitor such as allyl thiourea.
|
| |
|
|
The bioconcentration factor (BCF) is a measure of the tendency for a substance in water to accumulate in organisms, especially fish. The equilibrium concentration of a substance in fish can be estimated by multiplying its concentration in the surrounding water by its bioconcentration factor in fish. This parameter is an important parameter characterizing the food-chain and a determinant for human intake of aquatic food by the ingestion route.
|
| |
|
|
Ratio of tissue concentration to concentration in medium, with exposure only through the medium.
|
| |
|
|
The ability of a substance to be broken down into simpler substances by biological organisms such as bacteria or fungi or other natural physical processes (such as sunlight).
|
| |
|
Breakdown of a substance catalyzed by enzymes in vitro or in vivo. In ecotoxicology, it is almost en-
tirely due to microbial or fungal activity.
Note 1: Biodegradation may be classified for purposes of hazard assessment into three cate-
gories:
- Primary. Alteration of the chemical structure of a substance resulting in loss of a specific property of that substance.
- Environmentally acceptable. Biodegradation to such an extent as to remove unde-sirable properties of the compound. This often corresponds to primary biodegradation but depends on the circumstances under which the products are discharged into the environment.
- Ultimate. Complete breakdown of a compound to either fully oxidized or reduced simple molecules (such as carbon dioxide, methane, nitrate, ammonium, and (or) water).
Note 2: The products of biodegradation can be more harmful than the substance that was de-
graded.
|
| |
|
Movement of elements or molecules among organisms and nonliving compartments of the atmosphere,
lithosphere, and hydrosphere.
Note 1: Examples of biogeochemical cycles are the carbon, nitrogen, phosphorus, and sulfur cycles. These are defined as the global flow of C, N, P, and S atoms, respectively, from plants through animals to the atmosphere, soil, water, and back to plants.
Note 2: The water cycle refers to the global flow of water from surface and ground water sources to soil, plants, animals, and the atmosphere, and back to soil and surface water.
|
| |
|
|
A bioindicator (also biological indicator) is a measure, an index of measures, or a model that characterizes an ecosystem or one of its critical components. It may reflect biological, chemical or physical attributes of ecological condition. The primary uses of an indicator are to characterize current status and to track or predict significant change. With a foundation of diagnostic research, an ecological indicator may also be used to identify major ecosystem stress.
(Adapted from U.S. Environmental Protection Agency.)
|
| |
|
|
Extraction of metals from ores or soil by biological processes, mostly by microorganisms.
|
| |
|
Measuring hazardous substances in biologic materials (such as blood, hair, urine, or breath) to determine whether exposure has occurred. A blood test for lead is an example of biologic monitoring.
|
| |
|
The specific activity or capacity of a product to achieve a defined biological effect. Potency is the quantitative measure of biological activity.
|
| |
|
(in Toxicokinetics)
For a substance, the time required for the amount of that substance in a biological system to be reduced to one half of its value by biological processes, when the rate of the removal is approximately exponential.
|
| |
|
Refers to the process whereby certain substances such as pesticides or
heavy metals move up the food chain, work their way into rivers or
lakes, and are eaten by aquatic organisms such as fish, which in turn
are eaten by large birds, animals or humans. The substances become
concentrated in tissues or internal organs as they move up the chain.
|
| |
|
|
The field of mass spectrometry dealing with organic materials derived from biological systems. The spectrometry is different from organic mass spectrometry in that organic molecules are analyzed from the standpoint of biological function and the life sciences.
|
| |
|
|
continuous or repeated measurement of potentially toxic substances or their metabolites or biochemical effects in tissues, secreta, excreta, expired air or any combination of these in order to evaluate occupational or environmental exposure and health risk by comparison with appropriate reference values based on knowledge of the probable relationship between ambient exposure and resultant adverse health effects
|
| |
|
|
The increase in concentration of an element or compound that occurs in a food chain across trophic levels
|
| |
|
Ratio of concentrations of a compound at two consecutive trophic levels at steady state. It can also be
expressed in terms of a rate constant-based bioaccumulation model
B = Cn/Cn–1= αf/ke
where α is assimilation efficiency, f is feeding rate, and ke is the first-order elimination constant. B can be calculated from field data on assumed trophic relations or from laboratory feeding experiments.
See also: trophic enrichment factor.
|
| |
|
- Parameter that can be used to identify a toxic effect in an individual organism and can be used in extrapolation betweeen species;
- Indicator signalling an event or condition in a biological system or sample and giving a measure of exposure, effect ot susceptibility
|
| |
|
The dry weight estimation of organisms (usually microorganisms) in a given habitat or medium.
|
| |
|
|
methylation of a substance by enzymes in vitro and in vivo
|
| |
|
|
A compound that occurs naturally in a biological system. It can be a small molecule (sugar, lipid, sterol, amino acid, nucleoside, nucleotide, peptide) or a large molecule (protein, enzyme, polysaccharide, DNA, RNA).
|
| |
|
|
A polymer that occurs naturally in a biological system, including polypeptides, proteins, DNA, RNA and oligosaccharides.
|
| |
|
Use of living organisms such as microbes, fungi, plants or their enzymes to clean up oil spills or remove other
pollutants from soil, water, or wastewater; use of organisms such as
non-harmful insects to remove agricultural pests or counteract diseases
of trees, plants, and garden soil.
|
| |
|
|
A device that uses specific biochemical reactions mediated by isolated enzymes ,
immunosystems, tissues, organelles or whole cells to detect chemical
compounds, usually by electrical, thermal or optical signals.
|
| |
|
|
term used to describe the property of biomass to retain ions, mainly of heavy metals and radionuclides. The biosorption mechanisms, which are complex and poorly understodd, depend on whether the organisms are living or dead, the type of substrate, and the elemental species.
|
| |
|
Plants and animals in an environment. Some of these plants and animals
might be sources of food, clothing, or medicines for people.
Source: ATSDR Glossary of Terms
|
| |
|
|
Biotechnology generally refers to techniques used to modify the products of living organisms to improve plants or animals orv to develop useful microorganisms. IUn this sense, biotechnology has been practiced for centuries since people used yeast and bacteria to produce breads, wines, and chesses.
Today, the raspidly advancing field of biotechnology refers to the generation of unique organisms with new traits or new reproductive potential using cell and tissue cultures, cell fusion, molecular biology, and recombinant DNA technology.
|
| |
|
|
Chemical conversion of a substance that is mediated by living organisms or enzyme preparations derived therefrom.
|
| |
|
|
A bismuth salt of salicylic acid. Little absorbed from the
gastrointestinal tract, bismuth subsalicylate exerts a local effect on
the gastric mucosa, coating it and protecting it from the corrosive
effects of acid and pepsin. This agent also has local antimicrobial
properties.
|
| |
|
|
Blackfoot disease (BFD) is a severe form of peripheral vascular disease
(PVD), in which the blood vessels in the lower limbs are severely
damaged, resulting eventually in progressive gangrene. It has been
observed in certain areas along the southwestern coast of Taiwan where the average concentration of
dissolved arsenic in the wells is 671 ± 149 µg/L with an average
As(III)/As(V) ratio of 2.6. The first symptons of the disease are the
spottet discoloration of the skin on the feet that turns from brown to
black as the disease develops until amputation of the affected
extremities, the final resort to save the BFD victims, becomes
necesaary.
|
| |
|
|
Blackfoot disease (BFD) is a severe form of peripheral vascular disease (PVD),
in which the blood vessels in the lower limbs are severely damaged, resulting
eventually in progressive gangrene. It has been observed in Taiwan.
|
| |
|
a solution that does not contain a detectable amount of the analyte of interest. The blank solution is typically used for calibration purposes. Depending on its purpose the following blank solutions can be defined:
- Calibration blank (the solution used for creating the zero concentration point of the calibration graph; this solution contains only the diluent used for making the standard solution)
- Reagent blank (a blank solution that contains the reagents used to dissolve the samples such as acids used for digestion; the reading for this solution is typically substracted from sample readings)
- Method blank (a blank solution that has been handled similar to a sample, and to which the same reagents have been added, that had contact to the same type of vessels and that was treated by a similar procedure. This solution than is handled to monitor any type of contamination taking place with the method used.
The correct treatment of the analytical result for the blank solution does have a significant effect on the correctness of analytical results, especially for samples having analyte concentrations close to the limit of quantitation. Especially the measurement of the reagent and method blank must be well differentiated from the baseline correction.
|
| |
|
|
a) Tilt of an echellette with respect to the plane of a diffraction grating. b) Angle of incidence and diffraction at which the efficiency of a grating is maximum i.e. where light intensity diffracted/incident intensity is maximum.
|
| |
|
|
The loss of material from a column or septum caused by hightemperature operation. Bleed can result in ghost peaks and increased detector baseline offset and noise.
|
| |
|
Clinical trial technique in which, to eliminate bias in a research study, subjects (and sometimes clinical investigators [double blind]) remain unaware of which therapeutic approach (for example, investigational product or standard treatment) is provided.
|
| |
|
To protect the brain from infection and from damage that could be caused by foreign chemicals, the endothelial cell linings of its capillaries are tighly packed together. Nothing but water and nutrients that are actively transported by cellular mechanisms can pass through.
|
| |
|
Transfer of nucleic acids or proteins from an electrophoretic gel strip to a chemically reactive paper or membrane (such as nitrocellulose paper) or matrix (nylon,, for5 example) - to which they bind. Blotting is achieved through capillary diffusion (when the gel is placed between the paper or matrix and an absorptive pad) or through electrophoresis (electroblotting). Of the three types of blots, Southern hybridization (or Southern blot) transfers DNA; Northern blots transfer RNA, and Western blots transfer proteins (also called protein blots).
|
| |
|
|
Blunders are simply mistakes that occur on occasion and
produce erroneous results that are outliers and may be recognized as
such by statistical procedures. They cannot be treated by statistics.
|
| |
|
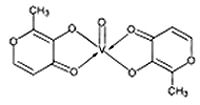 Bis(maltolato)oxovanadium(IV) (BMOV) can be synthesized by simple metathesis of vanadyl sulfate trihydrate and maltol (3-hydroxy-2-methyl-4-pyrone) (1:2). The ligand itself is commercially available and is an approved food additive in many countries, including Canada, the United Kingdom, and the United States. BMOV can be prepared in >90% yield in water, has a molecular weight of 317 and is soluble (millimolar scale) in a number of organic solvents as well as water. These properties together (neutral charge and aqueous solubility) contribute to high oral bioavailability. With these considerations in mind, the pentacoordinate, oxovanadium(IV) complex was developed, specifically as a potential insulin-mimetic agent. Bis(maltolato)oxovanadium(IV) (BMOV) can be synthesized by simple metathesis of vanadyl sulfate trihydrate and maltol (3-hydroxy-2-methyl-4-pyrone) (1:2). The ligand itself is commercially available and is an approved food additive in many countries, including Canada, the United Kingdom, and the United States. BMOV can be prepared in >90% yield in water, has a molecular weight of 317 and is soluble (millimolar scale) in a number of organic solvents as well as water. These properties together (neutral charge and aqueous solubility) contribute to high oral bioavailability. With these considerations in mind, the pentacoordinate, oxovanadium(IV) complex was developed, specifically as a potential insulin-mimetic agent.
|
| |
|
|
The total amount of a substance in the body. Some substances build up in the body because they are stored in fat or bone or because they leave the body very slowly.
|
| |
|
A mechanism through which atoms, ions, or group of atoms are held together in a molecule.
|
| |
|
|
A bonded phase (in chromatography) is a stationary phase which is covalently bonded to the support particles or to the inside wall of the column tubing.
|
| |
|
|
relatively coarse uncombusted or partly combusted residue of incineration that accumulates on the grate of a furnace
|
| |
|
|
Observation of a periodically-generated signal at a controlled time
after each repetition is initiated. By averaging many repetitions at a
given delay after event initiation, one obtains a more precise
measurement of the behavior of the repetitive event's time course than
could be obtained with a single observation.
|
| |
|
BrO3-. An inorganic anion, bromate is tasteless and colourless,
with a low volatility. As a moderately strong oxidant, bromate is reactive.
Normally, bromate is not found in nature, but it is a water disinfection
byproduct. It is formed during ozonation when ozone used to disinfect drinking
water reacts with (oxidises) naturally occurring bromide ions. Bromate is the
main cause for concern of ozonation as water treatment method. The amount of
bromate produced is influenced by the amount of (naturally occurring) bromide in
the water.
|
| |
|
Br-. An inorganic bromide anion. Also used to describe any compound
(usually a bromide salt) that contains the Br- ion, such as sodium bromide
(NaBr).
Inorganic bromide is widely distributed in the environment. It is commonly
found in natural waters, that is, streams, lakes and reservoirs. Br- comes from
wastewater discharges, agricultural run-off (mainly from soil fumigation) and
run-off from roads. The bromide ion is a precursor to water disinfectant by
products when ozone or possibly hypochlorite (bleach) are used as disinfectants,
and therefore needs to be controlled. The bromide ion itself is sometimes used
as a water disinfectant because, in water it forms hypobromous acid (HOBr)
considered as a strong disinfectant.
|
| |
|
|
A solution that maintains constant pH by resisting changes in pH from dilution or addition of small amounts of acids and bases.
|
| |
|
|
A quantitative measure of the potential of a buffer solution (defined as the number of equivalents of strong acid or base to cause a one pH unit change in 1 L of a buffer solution) or simply the ability of a buffer to withstand injections of a buffered sample solution without changing mobile-phase pH; capacity determined by pH, buffer pKa, and buffer concentration.
|
| |
|
Chemical analysis of an entire sample body (rock, soil) or a sub part with little or no segregation of specific areas or components.
|
| |
|
|
introduction of a butyl group into a compound
see also: alkylation
|
| |
|
|
Ionic species formed as a result of secondary reactions that take place in a collision/reaction cell. Also refer to secondary (side) reactions.
|
| |
|