Speciation analysis is often considered to be complex, prone to all kind of problems and therefore only manageable by experts. While indeed speciation analysis is more complex than trace element analysis, it shares the same type of error sources with many other analytical methodologies such as organic compound analysis.
Steps of chemical speciation analyisSpeciation analysis is a type of chemical analysis interested in the distribution of certain elements over different elemental species. Often but not always the interest is in trace elements. Speciation analysis therefore is not very special with respect to typical error sources but mostly shares common error sources also present in trace analysis and/or organic analysis. However, since speciation analysis provides a higher level of information value compared to trace element analysis, the possible errors are also more numerous. Errors may occur in all steps of chemical analysis:
- Project planning
- Sample collection
- Sample Storage
- Sample Preparation
- Analyte Separation
- Measurement
- Data Evaluation
- Reporting
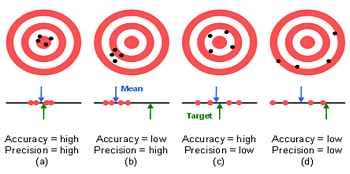
Errors in speciation analysis can both be stochastic in nature (random error) as well as systematic (bias, artefact). Random errors reduce the obtainable precision, while systematic errors compromise the accuracy. Both error types enhance the level of uncertainty.
Random errors are caused by unpredictable fluctuations in the readings of a measurement apparatus, or in the experimenter's capacity to reproduce the sample presentation to the apparatus. Such errors can in general be minimized by applying well known concepts to obtain high precision measurements, such as using highly reproducible weighing instead of imprecise volumetric measurements.
Systematic errors are predictable, and typically constant or proportional to the true value. If the cause of the systematic error can be identified, then it can usually be eliminated. Typical systematic errors in speciation analysis are:
- Contamination of the sample by the sampling procedure or later during analysis
- Analyte loss by adsorption onto solid parts of the sample, the sample container or the analytical apparatus.
- Incomplete extraction of a target species from the sample matrix
- Species transformation during analysis
- Incorrect identification of a species
- Unknown or “missing” species because separation (chromatography) is insufficient.
- Matrix effects (salts, organics, particulates) which suppress or enhance signal, or affect detection sensitivity or recovery.
- Non-spectral interferences (e.g. transport efficiency issues, adsorption losses, suppression of signal) often get ignored.
- Spectral interferences when using ICP-MS or related methods (e.g. polyatomics, isobaric overlaps) unless special care (collision/reaction gases, bandpass settings, dual detection) is taken.
- Calibration errors
Error sources have to be evaluated and resulting uncertainty has to be minimized by rigorous quality control and assurance (QA/QC), starting with appropriate method selection, method development and validation (for more details see:
Brief summary: Speciation Analysis - Striving for Quality).
Project planningA meaningful analysis starts with appropriate planning. Chemical analysis is performed in order to provide information that should answer a particular question. Depending on the application field (Environmental analysis, Clinical analysis, Food Analysis, Material Science, etc.) questions most often are related to one of the following topics:
- product identity, quality and safety for the consumer
- process efficiency with respect to raw materials, energy and waste production
- safety for the production plant and for the workplace environment
- absence of risks for the environment and its inhabitants
- emission to the environment, distribution and fate in the environment
- compliance with rules and legislation
- uptake, metabolism, accumulation and excretion by organisms
- ….many others
The analytical task should not be performed without knowing the questions to be answered. The analytical method should then be chosen in order to provide meaningful information answering the given question. Depending on the origin of the sample (environmental compartment, human body, food, industrial product) such questions are related to the specific activity of the target chemical species, such as:
- biological activity
- toxicity
- mobility
- bioavailability
- lifetime, fate and metabolism
- chemical and physical activity.
Since these characteristics are species related, data on the presence of elements and their total concentration do not promote relevant information (see
Brief summary: What is the use for total element concentrations ?). The real value of analytical tools is clearly related to the quality of information provided and the costs involved. Therefore the selection of an analytical methodology that cannot answer the question is the worst case with respect to costs and the biggest error.
 Related studies discussing typical errors in speciation analysis
Related studies discussing typical errors in speciation analysis

Stephen L.R. Ellison, William A. Hardcastle,
Causes of error in analytical chemistry: results of a web-based survey of proficiency testing participants, Accred. Qual. Assur., 17 (2012) 453-464.
DOI: 10.1007/s00769-012-0894-2

M.B. de la Calle, H. Emteborg, T.P.J. Linsinger, R. Montoro,
J.J.
Sloth, R. Rubio, M.J. Baxter,
J. Feldmann, P. Vermaercke, G. Raber,
Does the determination of inorganic arsenic in rice depend on the method?, Trends in Analytical Chemistry, 30/4 (2011) 641-651.
doi: 10.1016/j.trac.2010.11.015  J. Meija
J. Meija, L. Ouerdane, Z. Mester,
Isotope scrambling and error magnification in multiple-spiking isotope dilution, Anal. Bioanal. Chem., 394 (2009) 199.
doi:10.1007/S00216-009-2619-X Kevin A. Francesconi
Kevin A. Francesconi,
Michael Sperling,
Speciation analysis with HPLC-mass spectrometry: time to take stock, Analyst (London), 130/7 (2005) 998-1001.
doi: 10.1039/b504485p
H. Emons,
Artefacts and facts about metal(loid)s and their species from analytical procedures in environmental biomonitoring, Trends Anal. Chem. (Pers. Ed.), 21/6-7 (2002) 401-411.
doi: 10.1016/S0165-9936(02)00606-4

Hubert Chassaigne,
Ryszard Lobinski,
Detection of artefacts and peak identification in reversed-phase HPLC of metallothioneins by electrospray mass spectrometry, Talanta, 48/1 (1999) 109-118.
doi: 10.1016/S0039-9140(98)00220-3
Gemma Rauret, R. Rubio,
Sources of error in speciation analysis, Quim. Anal. (Barcelona), 16/S.1 (1997) S119-S130.
 H. Hintelmann
H. Hintelmann, R. Falter, G. Ilgen, R.D. Evans,
Determination of artifactual formation of monomethylmercury (CH3Hg+)
in environmental samples using stable Hg²+ isotopes with ICP-MS
detection: Calculation of contents applying species specific isotope
addition, Fresenius J. Anal. Chem., 358/3 (1997) 363-370.
doi: 10.1007/s002160050431
Philip Ekow Gardiner,
Factors that Influence the Distribution of Trace Element-Containing Chemical Species in Living Systems before and after Sample Collection, J. Trace Elem. Electrol. Health Dis., 7 (1993) 1-8.
 Related EVISA Resources
Related EVISA Resources Brief summary: Certified Reference Materials for Speciation Analysis
Brief summary: Certified Reference Materials for Speciation Analysis Brief summary: What is the use for total element concentrations ?
Brief summary: What is the use for total element concentrations ? Brief summary: Sample preservation for speciation analysis - General recommendations
Brief summary: Sample preservation for speciation analysis - General recommendations Brief summary: Species transformation during speciation analysis
Brief summary: Species transformation during speciation analysis
 Brief summary: Speciation Analysis - Striving for Quality
Brief summary: Speciation Analysis - Striving for Quality  Materials Database
Materials Database Link page: All about QA/QC
Link page: All about QA/QC Link page: All about CRMs
Link page: All about CRMs Related EVISA News (about problems in speciation analysis)
Related EVISA News (about problems in speciation analysis)
 March 14, 2013: Chromate in food samples: an artefact of wrongly applied analytical methodology
March 14, 2013: Chromate in food samples: an artefact of wrongly applied analytical methodology
last time modified: September 14, 2025