Species transformation is a special problem for speciation analysis, since changing speciation during analysis may bias or even corrupt the analytical result.
Background:Since many species have a limited stability and are in equilibrium to each other and are further related to the chemical environment (pH, pE, T, ...), species transformation may occur when the chemical environment is changing.
Species transformation The distribution concentration of chemical species in a sample is
dictated by the physical and chemical properties of the matrix. As such,
when a sample is pre-treated, in any way, there is a potential for
redistribution of homologous species. Therefore, species transformation may occur during all steps of speciation analysis, especially in those changing the chemical properties.
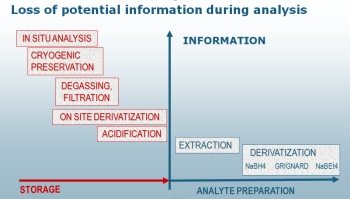
The more the chemistry is changing the more likely are species trans-formations destroying the original information on the species distribution (see the figure to the left).
Any addition of chemicals to the original sample (preservation agent, derivatization agents, complexing compounds, acids, solvents etc.) might influence the original species distribution. Further any changes in physical conditions such as temperature, pressure or radiation flux (such as light, X-ray etc.) might also change the chemical environment.
Species transformation has been observed during following steps (see the literature below):
- Sample collection and sample storage (e.g. redox reactions due to changing oxygen saturation)
- Sample drying and grinding (due to heating)
- Analyte extraction (e.g. methylation of mercury from sediments)
- Analyte derivatization (formation of methyl- and ethyl-mercury compounds from inorganic mercury during derivatization using sodium tetra(n-propyl)borate)
- Analyte separation
- by HPLC (Sb and As species transformation due to oxidation by oxygen in the mobile phase, interaction of species with the mobile phase)
- by CE (coenzyme-B12 was rapidly degraded in less than 2 h)
- by GC (Conversion of Phenylmercuric Salts to Diphenylmercury)
- Detection
- by ESI-MS (due to redox reactions at the ESI interface)
- by XANES (due to redox reactions induced by radiation)
In order to avoid such species transformation the following general recommendations can be given:
- Keep the sample preparation as short as possible with respect to steps and duration.
- Keep the use of chemicals to the absolute minimum.
- Avoid harsh conditions with respect to temperature and oxidation potential.
- If possible do not change the chemical conditions with respect to pH and pE.
- Analyse the sample as fast as possible.
Species degradation and sources of analytical error can be detected with the use of a
certified reference material (CRM). The CRM should be representative of the sample being analyzed and will serve to validate the analytical methodology. In case that species transformation is unavoidable, it can be corrected if the level of transformation can be monitored. A method for monitoring and correcting species transformation during analysis is provided by the
speciated isotope dilution analysis. The method is based on isotope dilution analysis, requiring an isotope standard for each species to be monitored. The isotope standard has to be in the exact form of the species to be monitored. Unfortunately this technique cannot be used for
mono-nuclidic elements such as arsenic and is further limited by the high costs of isotope standards and the limited availability of speciated compounds.
Further information can be found in the following chapters:
 Studies investigating species transformations during the analysis
Studies investigating species transformations during the analysis

Eri Matsumoto, Shihomi Ito, Tsutomu Nishimura,
Identification of Monomethyl-mono-thioarsonic Acid as the Major Thioarsenical Generated During Extraction Processes for Arsenic Species Analysis, Mar. Biotechnol., 25 (2023) 677–682.
DOI: 10.1007/s10126-023-10200-z

Vera Kantorová, Antonín Kana, Gabriela Krausová, Ivana Hyrslová, Oto Mestek,
Effect of protease XXIII on selenium species interconversion during their extraction from biological samples, J. Food Compos. Anal., 105 (2022) 104260.
DOI: 10.1016/j.jfca.2021.104260
Yifan Wang, Shuangyang Chen, Donglu Fang, Chunli Song, Liyan Zhao,
Ultrasonic treatment of arsenic species: Transformation kinetics analysis, Microchemical Journal 157 (2020) 105068.
DOI: 10.1016/j.microc.2020.105068

S.Y. Chen, B.M. Kimatu, D. Fang, X. Chen, G. Chen, Q. Hu, L. Zhao,
Effect of ultrasonic treatment on transformations of arsenic species in edible mushrooms, Anal. Lett. 53 (2020) 102–121.
DOI: 10.1080/00032719.2019.1639056

Jing Sun, Li Ma, Zhaoguang Yang, Lin Wang,
Optimization of species stability and interconversion during the complexing reaction for chromium speciation by high-performance liquid chromatography with inductively coupled plasma mass spectrometry, J. Sep. Sci., 37 (2014) 1944–1950.
DOI: 10.1002/jssc.201400170

Lihui Huang, Zhihua (Tina) Fan, Chang Ho Yu, Philip K. Hopke, Paul J. Lioy, Brian T. Buckley, Lin Lin, Yingjun Ma,
Interconversion of Chromium Species During Air Sampling: Effects of O3, NO2, SO2, Particle Matrices, Temperature, and Humidity, Environ. Sci. Technol. 2013, 47, 4408−4415.
DOI: 10.1021/es3046247

Daniel S. Alessi, Benjamin Uster, Camelia N. Borca, Daniel Grolimund, Rizlan Bernier-Latmani,
Beam-induced oxidation of monomeric U(IV) species, J. Synchrotron Rad., 20 (2013) 197–199.
doi:10.1107/S0909049512041763

Sameer Amereih, Thomas Meisel, Zaher Barghouthi, Wolfhard Wegscheider,
Determination of Inorganic Antimony Species Conversions during Its Speciation Analysis in Soil Using Isotope Dilution Techniques, J. Anal. Sci. Methods Instrum., 3 (2013) 130-136.
DOI: 10.4236/jasmi.2013.32016
Valerio B. Di Marco, Luca Raveane, Annalisa Dean and Pietro Traldi,
Perturbations produced by electrospray ionization mass spectrometry in the speciation of aluminium(III)/1,6-dimethyl-4-hydroxy-3-pyridinecarboxylate aqueous solutions, Rapid Commun. Mass Spectrom., 24 (2010) 868–874.
DOI: 10.1002/rcm.4457
Elham Zeini Jahromi, Wade White, Qiao Wu, Raghav Yamdagni,
Jürgen Gailer, Remarkable effect of mobile phase buffer on the SEC-ICP-AES derived Cu, Fe and Zn-metalloproteome pattern of rabbit blood plasma, Metallomics, 2 (2010) 460–468.
DOI: 10.1039/c003321a
Dong Nguyen Van, Thuy Thi Xuan Bui, Solomon Tesfalidet,
The transformation of phenyltin species during sample preparation of biological tissues using multi-isotope spike SSID-GC-ICPMS, Anal. Bioanal. Chem., 392 (2008) 737–747.
DOI: 10.1007/s00216-008-2316-1
Zoyne Pedrero, Jorge Ruiz Encinar, Yolanda Madrid,
Carmen Cámara,
Application of species-specific isotope dilution analysis to the correction for selenomethionine oxidation in Se-enriched yeast sample extracts during storage, J. Anal. At. Spectrom., 22/9 (2007) 1061-1066.
doi: 10.1039/b704807f
Oscar Palacios,
Ryszard Lobinski,
Investigation of the stability of selenoproteins during storage of human serum by size-exclusion LC-ICP-MS, Talanta, 71/4 (2007) 1813-1816.
doi: 10.1016/j.talanta.2006.08.018

Makoto Ichikawa, Nagatoshi Ide, Kazuhisa Ono,
Changes in Organosulfur Compounds in Garlic Cloves during Storage, J. Agri. Food Chem., 54/13 (2006) 4849-4854.
doi: 10.1021/jf060083o

Jen-How Huang, Gunter Ilgen,
Factors affecting arsenic speciation in environmental samples: sample drying and storage, Int. J. Environ. Anal. Chem., 86/5 (2006) 347-358.
doi: 10.1080/03067310500227878
Jen-How Huang,
Artifact formation of methyl- and ethyl-mercury compounds from inorganic mercury during derivatization using sodium tetra(n-propyl)borate, Anal. Chim. Acta, 532 (2005) 113–120.
doi:10.1016/j.aca.2004.10.057
Dong Nguyen Van, Richard Lindberg,
Wolfgang Frech,
Redistribution reactions of butyl- and phenyltin species during storage in methanol, J. Anal. At. Spectrom., 20/4 (2005) 266-272.
doi: 10.1039/b416355a
Maurizio Pettine, Silvio Capri,
Digestion treatments and risks of Cr(III)–Cr(VI) interconversions during Cr(VI) determination in soils and sediments—a review, Analytica Chimica Acta 540 (2005) 231–238.
doi: 10.1016/j.aca.2005.03.040
Valbona Celo, Ram V. Ananth, Susannah L. Scott, David R.S. Lean,
Methylmercury artifact formation during solid-phase extraction of water samples using sulfhydryl cotton fiber adsorbent, Anal. Chim. Acta, 516 (2004) 171–177.
doi:10.1016/j.aca.2004.04.032
Hendrik Emons,
Artefacts and facts about metal(loid)s and their species from analytical procedures in environmental biomonitoring, Trends Anal. Chem., 21/6+7 (2002) 401-411.
doi: 10.1016/S0165-9936(02)00606-4
Koen De Cremer, Rita Cornelis, Karel Strijckmans, Richard Dams, Norbert Lameire, Raymond Vanholder,
Behaviour of vanadate and vanadium-transferrin complex on different anion-exchange columns. Application to in vivo 48V-labelled rat serum, J. Chromatogr. B, 775/2 (2002) 143-152.
doi: 10.1016/S1570-0232(02)00278-7

I. Nukatsuka, Y. Shimizu, K. Ohzeki,
Determination of V(IV) and V(V) by Electrothermal AAS Following Selective Solid-Phase Extraction and the Study on the Change in the Oxidation State of Vanadium Species in Seawater during Sample Storage, Anal. Sci., 18/9 (2002) 1009.
doi: 10.2116/analsci.18.1009

Donald S. Ross, Heidi C. Hales, Grace C. Shea-McCarthy, and Antonio Lanzirotti,
Sensitivity of Soil Manganese Oxides: Drying and Storage cause Reduction, Soil Sci. Soc. Am. J., 65 (2001) 736-743.
doi:10.2136/sssaj2001.653736x
Donald S. Ross, Heidi C. Hales, Grace C. Shea-McCarthy, and Antonio Lanzirotti,
Sensitivity of Soil Manganese Oxides: XANES Spectroscopy May Cause Reduction, Soil Sci. Soc. Am. J., 65 (2001) 744–752.
doi:10.2136/sssaj2001.653744x

U.M. Grüter, M. Hitzke, J. Kresimon,
A.V. Hirner,
Derivatization of organometal(loid) species by sodium borohydride Problems and solutions, J. Chromatogr. A, 938 (2001) 225–236.
doi: 10.1016/S0021-9673(01)01342-5
Chad R. Hammerschmidt, William F. Fitzgerald,
Formation of Artifact Methylmercury during Extraction from a Sediment Reference Material, Anal. Chem., 73 (2001) 5930-5936.
doi: 10.1021/ac010721w Holger Hintelmann
Holger Hintelmann,
Comparison Of Different Extraction Techniques Used For Methylmercury Analysis With Respect To Accidental Formation Of Methylmercury During Sample Preparation, Chemosphere, 39/7 (1999) 1093-1105.
doi: 10.1016/S0045-6535(99)00180-0,

Vahid Majidi, Nancy J. Miller-Ihli,
Potential sources of error in capillary electrophoresis–inductively coupled plasma mass spectrometry for chemical speciation, Analyst, 123 (1998) 809–813.
DOI: 10.1039/A708256H

J.P. Snell, J. Qian, M. Johansson, K. Smit, W. Frech,
Stability and reactions of
mercury species in organic solution. Analyst, 123/5 (1998) 905−909. DOI:
10.1039/A708391B
O. Heudi, A. Cailleux, R Allain,
Interactions Between Cisplatin Derivatives and Mobile Phase During Chromatographic Separation, Chromatographia 44/1-2 ( 1997)19-24.
doi: 10.1007/BF02466510
Ronald C. Dressman,
The Conversion of Phenylmercuric Salts to Diphenylmercury and Phenylmercuric Chloride Upon Gas Chromatographic Injection, J. Chromatogr. Sci., 10/7 (1972) 468-472.
doi: 10.1093/chromsci/10.7.468 Related EVISA Resources
Related EVISA Resources
 Brief summary: Error sources in speciation analysis - Overview
Brief summary: Error sources in speciation analysis - Overview Brief summary: Speciation analysis - Striving for Quality
Brief summary: Speciation analysis - Striving for Quality Brief summary: Sample preservation for speciation analysis - General recommendations
Brief summary: Sample preservation for speciation analysis - General recommendations Brief summary: Chemical speciation analysis for nutrition and food science
Brief summary: Chemical speciation analysis for nutrition and food science Brief summary: Chemical speciation analysis for the life sciences
Brief summary: Chemical speciation analysis for the life sciences Brief summary: Trace element speciation analysis for environmental sciences
Brief summary: Trace element speciation analysis for environmental sciences Brief summary: Standard methods for elemental speciation analysis
Brief summary: Standard methods for elemental speciation analysis Brief summary: Standard methods for tin speciation analysis
Brief summary: Standard methods for tin speciation analysis Brief summary: Standard methods for arsenic speciation analysis
Brief summary: Standard methods for arsenic speciation analysis Brief summary: Standard methods for chromium speciation analysis
Brief summary: Standard methods for chromium speciation analysis Brief summary: Standard methods for mercury speciation analysis
Brief summary: Standard methods for mercury speciation analysis Link database: Sample preservation/Storage
Link database: Sample preservation/Storage Related EVISA News (newest first)
Related EVISA News (newest first)
 January 15, 2008: Species-specific isotope dilution analysis has
been adopted as an official method under US legislation
January 15, 2008: Species-specific isotope dilution analysis has
been adopted as an official method under US legislation
 February 9, 2006: Preservation of arsenic speciation in natural water samples for up to three months
February 9, 2006: Preservation of arsenic speciation in natural water samples for up to three months last time modified: December 13, 2023