According to a new CDC study different organomercury compounds share the same fundamental mechanisms related to their toxicity. There is no harmless mercury, both methylmercury and ethylmercury are extremely toxic.
Background:
It is well known that toxicity of metal compounds depends on the species, therefore different mercury species differ both with respect to level of toxicity but also with respect to the action of toxicity and target organ. Organomercury compounds are well known for their neurotoxicity. Since one of its potent group members methylmercury is produced by microbial activity from inorganic mercury and then enters especially the aquatic food chain, consumers of seafood are regularly warned to limit the consumption of contaminated fish. Such warnings are especially addressed to pregnant women, since prenatal exposure is especially harmful for the development of the child.
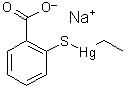
Structure of thimerosal
|
Another organomercury compound "Thimerosal" is used as preservative and adjuvant in some vaccines. Interestingly this compound is declared to be safe by the producers of the vaccines as well as public health organizations such as the CDC. While the compounds releases ethylmercury to the body, this compounds is declared to be totally different from methylmercury with respect to metabolism and toxicity. Now, two CDC scientists have published research dissenting the assertion that thimerosal is safe. As they conclude in their study, there is "no good mercury" and "bad mercury" but both species are equally poisonous to the brain.
The CDC researchers J.F. Risher and P. Tucker published their study last month in the journal "Reviews of Environmental Contamination and Toxicology". The 45-pages meta-review of relevant scientific publications examines the various ways that alkylmercury compounds harms the human body. The authors conclude that published studies reveal that there are some similarities between the mechanisms of the toxic action of the mono-alkyl mercury compounds methylmercury (MeHg) and ethylmercury (EtHg).:
- Methylmercury, the highly-regulated neurotoxin found in fish, and ethylmercury (found in medical products, including influenza and tetanus vaccines, ear drops and nasal sprays) are similarly toxic to humans. Methylmercury and ethylmercury share common chemical properties, and both significantly disrupt central nervous system development and function.
- Thimerosal is extremely toxic at very low exposures and is more damaging than methylmercury in some studies. For example, ethylmercury is even more destructive to the mitochondria in cells than methylmercury.
- The difference in manifested toxicity of MeHg and EtHg are likely the result of the differences in exposure, metabolism, and elimination from the body, rather than differences in mechanisms of action between the two.
CDC's website continues to feature now discredited safety assurances:
"Thimerosal contains ethylmercury, which is cleared from the human body more quickly than methylmercury, and is therefore less likely to cause any harm."
However, the new study makes the opposite conclusion:
"Thimerosal is quickly metabolized in vivo (in a living organism) due to its reactions with protein and non-protein thiols … so the effects of thimerosal reported in numerous articles are very likely the result of exposure to the metabolite ethylmercury."
The
CDC's webpage stubbornly insists that the "two types of mercury to which people may be exposed—methylmercury and ethylmercury—are very different." The new CDC study directly contradicts this assertion, "There are many commonalities/similarities in the mechanisms of toxic action of methylmercury and ethylmercury …"
The study meticulously details identical toxicity pathways shared by both forms of mercury:
- Both ethyl and methyl mercury cause DNA damage or impair DNA synthesis (Burke et al. 2006; Sharpe et al. 2012; Wu et al. 2008).
- Both cause oxidative stress/creation of reactive oxygen species (Dreiem and Seegal 2007; Garg and Chang 2006; Sharpe et al. 2012; Yin et al. 2007).
- Both decrease glutathione activity, thus providing less protection from the oxidative stress caused by MeHg and EtHg (Carocci et al. 2014; Ndountse and Chan (2008); Choi et al. 1996; Franco et al. 2006; Mori et al. 2007; Müller et al. 2001; Wu et al. 2008).
- Both cause effects on cell division by damaging the spindle apparatus during mitosis (Burke et al. 2006; Castoldi et al. 2000; Gribble et al. 2005; Kim et al. 2007; Ou et al. 1999; Machaty et al. 1999; Rodier et al. 1984).
- Both MeHg and EtHg bind to the amino acid cysteine (Clarkson 1995; Wu et al. 2008).
- Both MeHg and EtHg strongly inhibit the reacylation of arachidonic acid, thus inhibiting the reincorporation of this fatty acid into membrane phospholipids (Shanker et al. 2002; Verity et al. 1994; Zarini et al. 2006).
- Both cause an increase in NOS, causing an overproduction of NO (Chen et al. 2003; Chuu et al. 2001; Shinyashiki et al. 1998).
- Both disrupt glutamate homeostasis (Farina et al. 2003a, b; Manfroi et al. 2004; Mutkus et al. 2005; Yin et al. 2007).
- Both alter intracellular calcium homeostasis (Elferink 1999; Hare et al. 1993;Kang et al. 2006; Limke et al. 2004b; Machaty et al. 1999; Marty and Atchison1997; Minnema et al. 1987; Peng et al. 2002; Sayers et al. 1993; Sirois and Atchison, 2000; Szalai et al. 1999; Törnquist et al. 1999; Zarini et al. 2006).
- Both cause effects on receptor binding/neurotransmitter release involving one or more transmitters (Basu et al. 2008; Coccini et al. 2000; Cooper et al. 2003; Fonfria et al. 2001; Ida-Eto et al. 2011; Ndountse and Chan 2008; Yuan and Atchison 2003).
Despite this stark rejection of a decade of CDC
safety claims, CDC's public relations machine is still bucking the new
scientific consensus; the article concludes with a telling disclaimer in
tiny font:
"The findings and conclusions in this report
are those of the authors and do not necessarily represent the views of
the Agency for Toxic Substances and Disease Registry."
 The original study
The original study

John F. Risher and Pamela Tucker,
Alkyl Mercury-Induced Toxicity: Multiple Mechanisms of Action, Rev. Environ. Contam. Toxicol., 240 (2016) 105-149.
DOI 10.1007/398_2016_1 Related studies (in alphabetical order of the first author)
Related studies (in alphabetical order of the first author)

N. Basu, A.M. Scheuhammer, K. Rouvinen-Watt, R.D. EvansD, N. Grochowina, L.H.M. Chan,
The effects of mercury on muscarinic cholinergic receptor subtypes (M1 and M2) in captive mink, Neurotoxicology, 29 (2008) 328–334.
DOI: 10.1016/j.neuro.2008.01.003 
K. Burke, Y. Cheng, B. Li, A. Petrov, P. Joshi, R. Berman, K. Reuhl, E. DiCicco-Bloom E,
Methylmercury elicits rapid inhibition of cell proliferation in the developing brain and decreases cell cycle regulator, cyclin e. Neurotoxicology, 27/6 (2006)970–981.
DOI: 10.1016/j.neuro.2006.09.001
A. Carocci, N. Rovito, M.S. Sinicropi, G. Gehchi,
Mercury toxicity and neurodegenerative effects, Rev. Environ. Contam. Toxicol., 229 (2014) 1–18.
DOI: 10.1007/978-3-319-03777-6_1
A.F. Castoldi, S. Barni, I. Turin, C. Gandini, L. Manzo,
Early acute necrosis, delayed apoptosis and cytoskeletal breakdown in cultured cerebellar granule neurons exposed to methylmercury, J. Neurosci. Res., 59 (200) 775–787.
DOI: 10.1002/(SICI)1097-4547(20000315)59:6%3C775::AID-JNR10%3E3.0.CO;2-T 
Y.J. Chen, H. Jiang, J. Quilley,
The nitric oxide- and prostaglandin-independent component of the renal vasodilator effect of thimerosal is mediated by epoxyeicosatrienoic acids, J. Pharmacol. Exp. Ther., 304/3 (2003) 1292–1298.
DOI: 10.1124/jpet.102.042671
B.H. Choi, S. Yee, M. Robles,
The effects of glutathione glycoside in methylmercury poisoning, Toxicol. Appl Pharmacol., 141/2 (1996) 357–364.
DOI: 10.1006/taap.1996.0300 
J.-J. Chuu, C.-J. Hsu, S.-Y. Lin-Shiau,
Abnormal auditory brainstem responses for mice treated with mercurial compounds: involvement of excessive nitric oxide, Toxicology, 162 (2001) 11–22.
DOI: 10.1016/S0300-483X(01)00348-1 
T.W. Clarkson,
Environmental contaminants in the food chain. Am. J. Clin. Nutr., 61/3 (1995) 682s–686s.
PMID: 7879738
T. Coccini, G. Randine, S.M. Candura, R.E. Nappi, L.D. Prockop, L. Manzo,
Low-level exposure to methylmercury modifies muscarinic cholinergic receptor binding characteristics in rat brain and lymphocytes: physiologic implications and new opportunities in biologic monitoring, Environ. Health Perspect., 108/10 (2000) 29–33.
PMCID: PMC1637867
J.R. Cooper, F.E. Bloom, R.H. Roth,
The biochemical basis of neuropharmacology, 8th edn., 2003, Oxford University Press, Oxford

A. Dreiem, R.F. Seegal,
Methylmercury-induced changes in mitochondrial function in striatal synaptosomes are calcium-dependent and ROS-independent, Neurotoxicol., 28 (2007) 720–726.
DOI: 10.1016/j.neuro.2007.03.004
J.G.R. Elferink,
Thimerosal: a versatile sulfhydryl reagent, calcium mobilize, and cell function-modulating agent, Gen Pharmacol 33 (1993) 1–6.
DOI: 10.1016/S0306-3623(98)00258-4

M. Farina, M.E.S. Frizzo, F.A.A. Soares, F.D. Schwalm, M.O. Detrich, G. Zeni, J.B.T. Rocha, D.O. Souza,
Ebselen protects against methylmercury-induced inhibition of glutamate uptake by cortical slices from adult mice, Toxicol. Lett., 144 (2003) 351–357.
DOI: 10.1016/S0378-4274(03)00242-X
M. Farina, K.C.S. Dahm, F.D. Schwalm, A.M. Brusque, M.E.S. Frizzo, G. Zeni, D.O. Souza, J.B.T. Rocha,
Methylmercury increases glutamate release from brain synaptosomes and glutamate uptake by cortical slices from suckling rat pups: modulatory effect of ebselen, Toxicol. Sci., 73 (2003) 135–140.
DOI: 10.1093/toxsci/kfg058
E. Fonfria, E. Rodrigues-Farre, C. Sunol,
Mercury interaction with the GABAA receptor modulates the benzodiazepine binding site in primary cultures of mouse cerebellar granule cells, Neuropharmacology 41/7 (2001) 819–833.
DOI: 10.1016/S0028-3908(01)00130-7
J.L. Franco, A. Teixeira, F.C. Meotti, C.M. Ribas, J. Stringari, S.C.G. Pomblum, A.M. Moro, D. Bohrer, A.V. Bairos, A.L. Dafre, A.R.S. Santos, M. Farina,
Cerebellar thiol status and motor deficit after lactational exposure to methylmercury, Environ Res 102 (2006) 22–28.
DOI: 10.1016/j.envres.2006.02.003
T.K. Garg, I.Y. Chang,
Methylmercury causes oxidative stress and cytotoxicity in microglia: attenuation by 15-deoxy-delat 12, 14-prostaglandin J2. J Neuroimmunol., 171/1–2 (2006) 17–28.
DOI: 10.1016/j.jneuroim.2005.09.007
E.J. Gribble, S.-W. Hong, E.M. Faustman,
The magnitude of methylmercury-induce cytotoxicity and cell arrest is p53-dependent, Birth Def. Res. A Clin. Mol. Teratol., 73/1 (2005) 29–38.
DOI: 10.1002/bdra.20104 
M.F. Hare, K.M. McGinnis, W.D Atchison,
Methylmercury increases intracellular concentrations of Ca++ and heavy metals in NG108-15 cells, J. Pharmacol. Exp. Ther., 266/3 (1993) 1626–1635.
M. Ida-Eto, A. Oyabu, T. Ohkawara, Y. Tashiro, N. Narita, M. Narita,
Prenatal exposure to organomercury, thimerosal, persistently impairs the serotonergic and dopaminergic systems in the rat brain: Implications for association with developmental disorders. Brain Dev., 35 (2013):261–264.
DOI: 10.1016/j.braindev.2012.05.004
M.S. Kang, J.Y. Jeong, J.H. Seo, H.J. Jeon, K.M. Jung, M-R. Chin, C-K. Moon, J.V. Bonventre, S.Y. Jung, D.K. Kim,
Methylmercury-induced toxicity is mediated by enhanced intracellular calcium through activation of phosphatidylcholine-specific phospholipase C, Toxicol. Appl. Pharmacol., 216 (2006) 206–215.
DOI: 10.1016/j.taap.2006.04.016
Y.-J. Kim, Y.-S. Kim, M.-S. Kim, J.-C. Ryu,
The inhibitory mechanism of methylmercury on differentiation of human neuroblastoma cells, Toxicology 234/1–2 (2007) 1–9.
DOI: 10.1016/j.tox.2007.01.003
T.L. Limke, J.J. Bearss, W.D. Atchison,
Acute exposure to methylmercury causes Ca2+ dysregulation and neuronal death in rat cerebellar granule cells through an M3 muscarinic receptor linked pathway, Toxicol. Sci., 80/1 (2004) 60–68.
DOI: 10.1093/toxsci/kfh131
Z. Machaty, W.H. Wang, B.N. Day, R.S. Prather,
Calcium release and subsequent development induced by modification of sulfhydryl groups in porcine oocytes, Biol. Reprod., 61 (1999) 1384–1391.
PMID: 10330097
C.B. Manfroi, F.D. Schwalm, V. Cereser, F. Abreu, A. Oliveira, L. Bizarro, J.B.T. Rocha, M.E.S. Frizzo, D.O. Souza, M. Faroma,
Maternal milk as methylmercury source for suckling mice: neurotoxic effects involved with the cerebellar glutamatergic system, Toxicol. Sci., 81 (2004) 172–178.
DOI: 10.1093/toxsci/kfh201
M.S. Marty, W.D. Atchison,
Pathways mediating Ca2+ entry in rat cerebellar granule cells following in vitro exposure to methyl mercury, Toxicol. Appl. Pharmacol., 147 (1997) 319–330.
DOI: 10.1006/taap.1997.8262
D.J. Minnema, G.P. Cooper, R.D. Greenland,
Effects of methylmercury on neuro-transmitter release from rat brain synaptosomes, Toxicol. Appl. Pharmacol., 99/3 (1987) 510–521.
DOI: 10.1016/0041-008X(89)90158-0
N. Mori, A. Yasutake, K. Hirayama,
Comparative study of activities in reactive oxygen species production/defense system in mitochondria of rat brain and liver, and their susceptibility to methylmercury toxicity, Arch Toxicol 81/11 (2007) 769–778.
DOI: 10.1007/s00204-007-0209-2
M. Müller, G. Westphal, A. Vesper, J. Bünger, E. Hallier,
Inhibition of the human erythrocytic glutathione-S-transferase T1 (GST T1) by thimerosal, Int. J. Hyg. Environ. Health, 203/5-6 (2001) 479–481.
DOI: 10.1078/1438-4639-00061
L. Mutkus, J.L. Aschner, T. Syversen, G. Shanker, U. Sonnewald, M. Aschner,
In vitro uptake of glutamate in GLAST- and GLT-1-transfected mutant CHO-K1 cells is inhibited by the ethylmercury-containing preservative thimerosal, Biol. Trace Elem. Res., 105/1–3 (2005) 71–86.
DOI: 10.1385/BTER:105:1-3:071 
L.T. Ndountse, H.M. Chan,
Methylmercury increases N-methyl-D-aspartate receptors on human SH-SY 5Y neuroblastoma cells leading to neurotoxicity, Toxicology, 249/2-3 (2008) 251-5.
DOI: 10.1016/j.tox.2008.05.011
Y.C. Ou, S.A. Thompson, R.A. Ponce, J. Schroeder, T.J. Kavanagh, E.M. Faustman,
Induction of the cell cycle regulatory gene p21 (WAF1, C1P1) following methylmercury exposure in vitro and in vivo, Toxicol. Appl. Pharmacol., 157/3 (1999) 203–212.
DOI: 10.1006/taap.1999.8685
S. Peng, R.K. Hajela, W.D. Atchison,
Effects of methylmercury on human neuronal L-type calcium channels transiently expressed in human embryonic kidney cells (HEK-293), J. Pharmacol. Exp. Ther., 302/2 (2002) 424–432.
DOI: 10.1124/jpet.102.032748 
P.M. Rodier, M. Aschner, P.R. Sager,
Mitotic arrest in the developing CNS after prenatal exposure to methylmercury, Neurobehav. Toxicol. Teratol., 6 (1984) 379–385.
PMID: 6514102
L.G. Sayers, G.R. Brown, R.H. Michell, F. Michelangeli,
The effects of thimerosal on calcium uptake and inositol 1,4,5-trisphosphate-induced calcium release in cerebellar microsomes, Biochem. J., 289 (1993) 883887.
DOI: 10.1042/bj2890883
G. Shanker, L.S. Matkus, S.J. Walker, M. Aschner,
Methylmercury enhances arachidonic acid release and cytosolic phospholipase A2 expression in primary cultures of neonatal astrocytes, Mol. Brain Res., 106 (2002) 1–11.
DOI: 10.1016/S0169-328X(02)00403-5
M.A. Sharpe, A.D. Livingston, D.S. Baskin,
Thimerosal-derived ethylmercury is a mitochondrial toxin in human astrocytes: possible role of Fenton chemistry in the oxidation and breakage of mtDNA, J. Toxicol., 2012 (2012) #373678.
DOI: 10.1155/2012/373678
M. Shinyashiki, Y. Kumagai, H. Nakajima, J. Nagafune, S. Homma-Takeda, M. Sagai, N. Shimojo,
Differential changes in rat brain nitric oxide synthase in vivo and in vitro by methylmercury, Brain Res., 798 (1998) 147–155.
DOI: 10.1016/S0006-8993(98)00400-4
J.E. Sirois, W.D. Atchison,
Methylmercury affects multiple subtypes of calcium channels in rat cerebellar granule cells, Toxicol. Appl. Pharmacol., 167 (2000) 1–11.
DOI: 10.1006/taap.2000.8967
G. Szalai, R. Krishnamurthy, G. Hajnoczky,
Apoptosis driven by IP3-linked mitochondrial calcium signals, EMBO J., 18/22 (1999) 6349–6361.
DOI: 10.1093/emboj/18.22.6349
K. Törnquist, P. Vainio, A. Titievsky, B. Dugué, R. Tuominen,
Redox modulation of intracellular free calcium concentration in thyroid FRTL-5 cells; evidence for an enhanced extrusion of calcium, Biochem. J., 339 (199) 621–628.
PMCID: PMC1220198
M.A. Verity, T. Sarafian, E.H.K. Pacifici, A. Sevanian, P
hospholipase A2 stimulation by MeHg in neuron culture, J. Neurochem., 62/2 (1994) 705–714.
DOI: 10.1046/j.1471-4159.1994.62020705.x
X. Wu, H. Liang, K.A. O’Hara, J.C. Yalowich, B.B. Hasinoff,
Thiol-modulated mechanisms of the cytotoxicity of thimerosal and inhibition of DNA topoisomerase H alpha. Chem. Res. Toxicol., 21/2 (2008) 483–493.
DOI: 10.1021/tx700341n
Z.B. Yin, D. Milatovic, J.L. Aschner, T. Syversen, J.B.T. Rocha, D.O. Souza, M. Sidoryk, J. Albrecht, M. Aschner,
Methylmercury induces oxidative injury, alterations in permeability and glutamine transport in cultured astrocytes, Brain Res., 1131/1 (2007) 1–10.
DOI: 10.1016/j.brainres.2006.10.070
Y. Yuan, W.D. Atchison,
Methylmercury differentially affects GABAA receptor-mediated spontaneous IPSCs in Purkinje and granule cells of rat cerebellar slices, J. Physiol., 550/1 (2003) 191–204.
DOI: 10.1113/jphysiol.2003.040543
S. Zarini, M.A. Gijon, G. Folco, R.C. Murphy,
Effect of arachidonic acid reacylation on leukotriene biosynthesis in human neutrophils stimulated with granulocyte-macrophage colony stimulating factor and formyl-methionyl-leucyl-phenylalanine, J. Biol. Chem., 81/15 (2006) 10134–10142.
DOI: 10.1074/jbc.M510783200 Related EVISA Resources
Related EVISA Resources Link database: Toxicity of Organic mercury compounds
Link database: Toxicity of Organic mercury compounds Brief summary: Speciation and Toxicity
Brief summary: Speciation and Toxicity  Link database: Human exposure to methylmercury via the diet
Link database: Human exposure to methylmercury via the diet  EVISA Advanced Search: All about Thimerosal
EVISA Advanced Search: All about Thimerosal  Link database: All about thimerosal (thiomersal)
Link database: All about thimerosal (thiomersal) Brief summary: Speciation analysis for the study of metallodrugs and their biomolecular interactions
Brief summary: Speciation analysis for the study of metallodrugs and their biomolecular interactions Related EVISA News
Related EVISA News May 5, 2014: Global policy on the use of mercury as a preservative in vaccine called discriminatory
May 5, 2014: Global policy on the use of mercury as a preservative in vaccine called discriminatory  September 12, 2013: Scientists reveal how organic mercury can interfer with vision
September 12, 2013: Scientists reveal how organic mercury can interfer with vision January 21, 2013: UNEP mercury treaty exempts vaccines for children
January 21, 2013: UNEP mercury treaty exempts vaccines for children  January 14, 2013: United Nations Global Mercury Treaty: Fifth and final session
January 14, 2013: United Nations Global Mercury Treaty: Fifth and final session  December 18, 2012: Pediatricians Argue to Keep Thimerosal in Some Vaccines
December 18, 2012: Pediatricians Argue to Keep Thimerosal in Some Vaccines
 October 12, 2012: Prenatal mercury intake linked to ADHD
October 12, 2012: Prenatal mercury intake linked to ADHD July 15, 2012: World Health Organization Fails In Its Effort To Defend Mercury In Vaccines Before United Nations
July 15, 2012: World Health Organization Fails In Its Effort To Defend Mercury In Vaccines Before United Nations
 June 19, 2012: Vaccine ingredient causes brain damage; some nutrients prevent it
June 19, 2012: Vaccine ingredient causes brain damage; some nutrients prevent it October 28, 2011: WHO worries mercury treaty could affect costs and availability of vaccines
October 28, 2011: WHO worries mercury treaty could affect costs and availability of vaccines  August 8, 2011: UNEP Global Mercury Treaty May Include Ban on Mercury in Medicine
August 8, 2011: UNEP Global Mercury Treaty May Include Ban on Mercury in Medicine June 19, 2011: Committee for Socio-economic Analysis agrees on
two draft opinions on restriction proposals for mercury compounds under
REACH
June 19, 2011: Committee for Socio-economic Analysis agrees on
two draft opinions on restriction proposals for mercury compounds under
REACH March 17, 2011: Researchers Urge the Removal of Mercury From Flu Shots
March 17, 2011: Researchers Urge the Removal of Mercury From Flu Shots  September 25, 2010: The European Chemical Agency (ECHA) calls for
comments on reports proposing restrictions on mercury and phenylmercury
September 25, 2010: The European Chemical Agency (ECHA) calls for
comments on reports proposing restrictions on mercury and phenylmercury August 16, 2010: Methylmercury: What have we learned from Minamata Bay?
August 16, 2010: Methylmercury: What have we learned from Minamata Bay? September 24, 2009: Huge field experiment for assessing human ethylmercury risk starting in october
September 24, 2009: Huge field experiment for assessing human ethylmercury risk starting in october July 15, 2009: New Study Finds: Thimerosal Induces Autism-like Neurotoxicity
July 15, 2009: New Study Finds: Thimerosal Induces Autism-like Neurotoxicity May 15, 2008: New study will investigate the influence of environmental factors in autism
May 15, 2008: New study will investigate the influence of environmental factors in autism May 3, 2006: Texas Study Relates Autism to Environmental Mercury
May 3, 2006: Texas Study Relates Autism to Environmental Mercury  March 24, 2006: Mercury Containing Preservative Alters Immune Function
March 24, 2006: Mercury Containing Preservative Alters Immune Function March 24, 2006: American lawmakers initiate mercury probe for vaccines
March 24, 2006: American lawmakers initiate mercury probe for vaccines April 27, 2005: New results about toxicity of thimerosal
April 27, 2005: New results about toxicity of thimerosal February 11, 2005: New findings about Thimerosal Neurotoxicity
February 11, 2005: New findings about Thimerosal Neurotoxicitylast time modified: July 22, 2020