|
EU restricts mercury fillings for children
(03.07.2018)
The use of silver fillings in baby teeth and children under the age of 15 is restricted in the EU since July 2nd, 2018.
EU directive:The EU directive, effective from July 1, places restrictions on the use of amalgam or ‘silver’ fillings in ‘baby’ teeth, and in children under 15, except when deemed strictly necessary on specific medical, including dental, needs. The directive is part of an EU push to reduce the release of mercury into the environment. It also restricts amalgam fillings for pregnant or breastfeeding women, again unless deemed necessary by the dental practitioner.
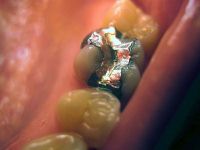 From January 2019 the use in dental amalgam will only be allowed as long as mercury is encapsulated. The text also imposes to dentists the use of devices to avoid mercury from ending up polluting water. The Commission will report by 30 June 2020 on the feasibility of a phase out of the use of mercury in dental amalgam for a later date, preferably by 2030. In the meantime, member states will prepare national plans to phase down the use of dental amalgam.
 Related information Related information
 Related EVISA News Related EVISA News (Newest first)
last time modified: July 3, 2018
|