|
Glossary
EVISA is providing a list of terms used in the area of speciation and fractionation analysis. Since speciation analysis is a field of analytical chemistry that is specified by a pronounced interdisciplinary cooperation between different sciences such as biochemistry, medicine, biology, environmental sciences, nutritional sciences and material sciences its terminology is a complex mixture of terms used in all these.
You may search for a term or browse the glossary alphabetically.
(In case that you cannot find the term you may consult more special glossaries or handbooks about nomenclature. For more details please consult EVISA's Link pages related to terminology,
|
|
Partition coefficient (KOA or POA) for a compound between octanol and air. Like Kow , it is a measure of lipophilicity.
|
| |
|
Ratio of the solubility of a chemical in octanol to its solubility in water at equilibrium ( Pow, Kow).
Note: Measure of lipophilicity, used in the assessment of both the uptake and physiological
distribution of organic chemicals and prediction of their environmental fate.
|
| |
|
|
Partition coefficient for a pollutant in the two-phase system octan-1-ol/water. Kow reflects the relative liophilicity of a pollutant and its potential for bioconcentration.
|
| |
|
An octapole is an octagonal array of cylindrical rods
that acts as an ion transmission device. An RF voltage and DC offset
voltage applied to the rods create an electrostatic field that transmits
the ions along the axis of the octapole rods.
(see also hexapole)
|
| |
|
Consists of eight parallel rods placed at equal distances from the center axis and with equal spacing
between the rods with an RF voltage applied to the rods in order to guide (increase the transmission of) ions through a volume of collision or reaction gas.
|
| |
|
|
The ORS is a a type of collision/reaction cell used in inductively coupled plasma mass spectrometry to remove interfering ions through ion/neutral reactions. As the name implies, the cell is using a octople instead of a quadruple. The octopole reaction system (ORS)) uses only helium or hydrogen and the
volume of the cell is smaller than that of a DRC. The small molecules
of helium and hydrogen collide with the large, unwanted polyatomic ions
formed in the plasma and break them up into other ions that can be
separated in the quadrupole mass analyser. However, unlike the DRC the
OCR system is based only on collision reactions and not on chemical
reactions.
|
| |
|
An odd-electron ion contains an unpaired electron; for example, [CH4+ ..]. The superscripted dot denotes
the unpaired electron. The molecular ion initially formed in electron ionization is an odd-electron ion.
  
  
|
| |
|
|
Occupational Exposure Limit: General term for
concentration of air contaminants above which people should not be
exposed at work.
|
| |
|
|
In oncolumn injection, sample enters the column directly from the syringe and does not contact other surfaces. On-column injection usually signifies cold injection for capillary columns.
|
| |
|
A chromatographic column, usually of capillary dimensions, in which the column wall, a liquid or an active solid supported on the column wall acts as the stationary phase.
Source: IUPAC
|
| |
|
|
An experimental procedure that is designed to measure a specific parameter, whereby the parameter is defined by this measurement procedure.
For example: Total alkalinity is operationally defined as the alkalinity neutralized by titration with a strong acid to the carbonic acid equivalence point. (IT = incremental titration, DIS = dissolved, TOT = total)
|
| |
|
|
An oral preparation of picoplatin, a third generation platinum compound
with antineoplastic activity. Designed to overcome platinum drug
resistance, picoplatin alkylates DNA, forming both inter- and
intra-strand cross-linkages, resulting in inhibition of DNA replication
and RNA transcription and the induction of apoptosis.
Because of the increase in steric bulk around the platinum center,
there is a relative reduction in the inactivation of picoplatin by
thiol-containing
species such as glutathione and metallothionein in comparison to
cisplatin.
|
| |
|
An ion trapping device that consists of an outer barrel-like electrode and a coaxial inner spindle like electrode that form an electrostatic field with quadro-logarithmic potential distribution. The frequency of harmonic oscillations of the orbitally trapped ions along the axis of the electrostatic field is independent of the ion velocity and is inversely proportional to the square root of m/z so that the trap can be operated as a mass analyzer using image current detection and Fourier
|
| |
|
|
Arsenic compounds containing carbon. They are mainly found in sea-living
organisms, although some of these compounds have also been found in species
living on land.
|
| |
|
|
Includes mercury complexes with organic ligands (e.g. humic/fulvic acids, amino acids, but without a Hg-carbon bond) and organic mercury bound via a carbon atom (CH3-HgOH, CH3HgCl, CH3HgCH3)
|
| |
|
|
Phosphorus fraction that has been formed primarily by biological processes. Organic phosphorus is converted to orthophosphate only by oxidative destruction of the organic matter (APHA, 1989). Total organic P maybe quantified by subtracting dissolved hydrolyzable phosphorus and orthophosphate values from total phosphorus (EPA, 1979). Dissolved organic P maybe quantified by subtracting dissolved hydrolyzable P and OPO4 from TDP result.
|
| |
|
|
Mercury compounds with a Hg-carbon bond (e.g. alkylmercury compounds).
|
| |
|
|
Compound with at least one covalent bond (sigma or pi) between a carbon atom and a metal atom. This term excludes other metal-organic complexes in which the metal is usually bound to nitrogen, oxygen, or phosphorus. It also excludes metaloids such as arsenic and selenium which can form covalent bonds with alkyl and aryl groups.
|
| |
|
|
The term is generally undestood to mean an organic derivative of phosphoric or similar acids. Many OPs inhibit an enzyme known as acetylcholinesterase, but not all OPs (e.g. glyphosate) demonstrate this effect. Some OPs react with other proteins such as neuropathy target esterase. Inhibitors of acetylcholinesterase affect certain nerve junctions in animals, as well as parasympathetic effector sites (the heart, lungs, stomach, intestines, urinary bladder, prostate, eyes and salivary glands). The transmission of impulses across nerve junctions involves the release of a transmitter chemical, which, in the case of many nerves, is acetylcholine. To stop the nerve continuing to transmit the message, the transmitter, acetylcholine, must be broken down immediately after it has had its effect. This breakdown is brought about by an enzyme, acetylcholinesterase. By inhibiting the enzyme acetylcholinesterase, OPs prevent the nerve junction from functioning properly. In humans, anticholinesterase OPs have broadly similar actions to those seen in other species. Acetylcholinesterase inhibition causes acute effects in humans and other mammals. The symptoms in humans, which generally occur when acetylcholinesterase activity has been reduced by about 50%, may include: headache, exhaustion and mental confusion together with blurred vision, sweating, salivation, chest tightness, muscle twitching and abdominal cramps. The severity of the effects depends on the degree of acetylcholinesterase inhibition.
|
| |
|
Organosilicon compounds are man-made organic compounds containing carbon silicon bonds. Organosilicon chemistry is the corresponding science exploring their properties and reactivity.
Like carbon, the organically bound silicon is tetravalent and tetrahedral. Carbon-silicon bonds are not found in any biomolecule.The first organosilicon compound, tetraethylsilane was discovered by Charles Friedel and James Crafts in 1863 by reaction of tetrachlorosilane with diethylzinc.
|
| |
|
|
Organotin compounds or stannanes are chemical compounds based on tin with hydrocarbon substituents. Organotin compounds are commercially applied as hydrochloric acid scavengers in polyvinyl chloride (PVC) and as biocides. Tributyltin oxide for example (or tributyltin for short) has been extensively used as a wood preservative. Tributyltin compounds are used as marine anti-biofouling agents. Concerns over toxicity of these compounds (some reports describe biological effects to marine life at a concentration of 1 nanogram per liter) have led to a world-wide ban by the International Maritime Organization.
|
| |
|
|
A platinum(IV) analogue with antineoplastic activity. Ormaplatin
alkylates DNA, forming both inter- and intra-strand platinum-DNA
crosslinks, which result in inhibition of DNA replication and
transcription and cell-cycle nonspecific cytotoxicity.
|
| |
|
|
Analytical strategies which employ combinations of various separation and/or detection methods are called orthogonal analytical concepts.
|
| |
|
– A water molecule is present between the surface functional group and the bound ion or molecule (Sposito 1989). Outer-sphere complexes involve electrostatic coulombic interactions. Outer-sphere complexation is usually a rapid process that is reversible, and adsorption occurs only on surfaces that are of opposite charge to the adsorbate
|
| |
|
|
An observation in a set of data which appears to be inconsistent with the remainder of that set.
|
| |
|
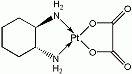 Oxaliplatin (trans-1-diaminocyclohexane oxalatoplatinum) is a platinum-based chemotherapy metallodrug in the same family as cisplatin and carboplatin. Compared to cisplatin the two amine groups are replaced . with 1,2-diaminocyclohexane (DACH) and with an oxalate ligand as a
'leaving group' for improved antitumour activity. A 'leaving group' is an atom or a group of atoms that
is displaced as a stable species taking with it the bonding electrons.
After displacement of the labile oxalate ligand leaving group, active
oxaliplatin derivatives, such as monoaquo and diaquo DACH platinum,
alkylate macromolecules, forming both inter- and intra-strand
platinum-DNA crosslinks, which result in inhibition of DNA replication
and transcription and cell-cycle nonspecific cytotoxicity. The DACH
side chain appears to inhibit alkylating-agent resistance.
CAS number: 61825-94-3
Chemical formula C8H14N2O4Pt
Molecular weight 397.3
|
| |
|
|
A chemical reaction in which a compound or radical loses electrons, that is in which the positive valence is increased.
|
| |
|
|
The oxidation number of an element in any chemical entity is the number of charges which would remain on a given atom if the pairs of electrons in each bond to that atom were assigned to the more electronegative member of the bond pair. The oxidation (Stock) number of an element is indicated by a roman numeral placed in parentheses immediately following the name (modified if necessary by an appropriate ending) of the element to which it refers. The oxidation number may be positive, negative or zero. Zero, not a roman numeral, is represented by the usual cipher, 0. The positive sign is never used. An oxidation number is always positive unless the minus sign is explicitly used. Note that it cannot be non-integral (see also mixed valency). Non-integral numbers may seem appropriate in some cases where a charge is spread over more than one atom, but such a use is not encouraged. In such ambiguous cases, the charge number, which designates ionic charge, can be used. A charge (Ewens-Bassett) number is a number in parentheses written without a space immediately after the name of an ion, and whose magnitude is the ionic charge. Thus the number may refer to cations or anions, but never to neutral species. The charge is written in arabic numerals and followed by the sign of the charge. In a coordination entity, the oxidation number of the central atom is defined as the charge it would bear if all the ligands were removed along with the electron pairs that were shared with the central atom. Neutral ligands are formally removed in their closed-shell configurations. Where it is not feasible or reasonable to define an oxidation state for each individual member of a group or cluster, it is again recommended that the overall oxidation level of the group be defined by a formal ionic charge, the net charge on the coordination entity.
|
| |
|