Korean researchers found that humic acids can reduce the lead toxicity for bacteria in contaminated soil
Background:Environmental toxicology emphasizes the difference from traditional toxicology in which pure compounds of interest are studied. When the objective is to study the fate and effects of trace elements in the environment, knowledge of the speciation of the elements and their physico-chemical forms is important. Risk-based contaminated site management uses the total amount of contaminant in contaminated environmental media, in particular, for organic contaminants, as a point of exposure; however, with heavy metals that could have different chemical forms in contaminated sites, the portion of heavy metals that actually contributes to toxicity (i.e., bioavailable portion) needs to be considered as a point of exposure in risk characterization. However, current risk assessment for contaminated soil does not consider bioavailability of heavy metals, which highly depends on physicochemical properties of environmental media.
With respect to risk assessment and legislation it becomes more and more clear that failure to consider properly chemical speciation of elements can lead to poor use of our resources. Therefore, an important goal in ecotoxicology is to predict the bioavailability of dissolved metals as a function of their speciation in the environment. Bioavailability of metals in contaminated sites is largely controlled by the physicochemical properties of the environmental media such as organic matter (OM), pH, cation exchange capacity, and oxidation-reduction potential. OM is one of the most influential factors controlling metal mobility, bioavailability, and toxicity. This can be assigned to its large number of functional groups such as carboxylic and phenolic hydroxyl groups that can readily interact with metal cations.
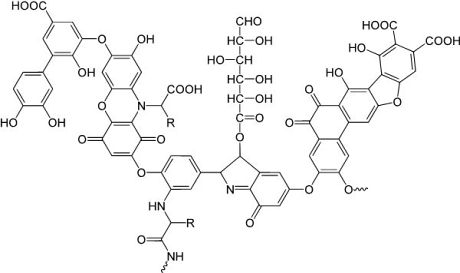
Figure 1:
Model
structure of a humic acid (one component of OM), having a variety of
components including quinone, phenol, catechol, and sugar moieties
:
In their new study Korean scientists have now investigated the effect of humic acid (HA), used as a surrogate of organic matter, on Pb toxicity and the subsequent effect on risk characterization in ecological risk assessment. Pb toxicity was assessed via Microtox® bacterial bioassay using Vibrio fischeri in the presence and absence of HA. HA was added to the Pb solution to prepare Pb-HA mixtures that have three chemical forms of Pb - Pb sorbed on particulate HA (pHA) and dissolved Pb, which consists of Pb complexed with dissolved HA (dHA) and free Pb ions. The mixtures were then incubated at room temperature for different periods (0-48 h) to see the effect of increasing contact period on Pb toxicity. Pb sorbed on pHA and dissolved Pb was separated by filtering the mixture through a 0.45 µm filter. Acute Pb toxicity was then determined using both the original mixture and the filtered solution to assess the contribution of the Pb sorbed on pHA and the dissolved Pb on the mixture toxicity.
The results showed that the dissolved Pb concentrations of the filtrates decreased with increasing contact time and the toxic effects decreased (the EC10 values increased). From this result the authors concluded that the Pb toxicity highly depends on the soluble fraction. Also, reduced Pb toxicity with increasing dHA concentrations, probably due to formation of Pb-dHA complexes, indicated that Pb toxicity largely comes from free Pb ions.
As a final conclusion, the authors emphasize that the incorporation of bioavailable heavy metal concentrations in environmental media as a point of exposure in ecological risk assessment is essential for more realistic risk assessment.
Comment:In 2007 we reported about a study investigating
the effect of humic acids on the toxicity of lead for marine invertebrates. An international research group was questioning the widely accepted "free ion activity model". According to their results, humic acids increase dissolved lead bioavailability for marine invertebrates. This seems to be an indication that bioavailability also depends on the element of interest, the target organism and the physicochemical characterization of the environmental media and cannot be generally assessed by in vitro experiments and transferred between different environmental compartments.
Michael Sperling
 The new study:
The new study:
Jinsung An, Eun Hea Jho, Kyoungphile Nam,
Effect of dissolved humic acid on the Pb bioavailability in soil solution and its consequence on ecological risk, J. Hazard. Mater., 286 (2015) 236-241.
doi: 10.1016/j.jhazmat.2014.12.016 Related studies
Related studies:

Jinsung An, Seulki Jeong, Hee Sun Moon, Eun Hea Jho, Kyoungphile Nam,
Prediction of Cd and Pb toxicity to Vibrio fischeri using biotic ligand-based models in soil, J. Hazard. Mater., 203–204 (2012) 69–76.
doi:10.1016/j.jhazmat.2011.11.085 
P.N. Williams, H. Zhang, W. Davison, A.A. Meharg, M. Hossain, G.J. Norton, H. Brammer, M.R. Islam,
Organic matter—solid phase interactions are critical for predicting arsenic release and plant uptake in Bangladesh paddy soils, Environ. Sci. Technol., 45 (2011) 6080–6087.
doi: 10.1021/es2003765
H. Ryu, J.S. Chung, T. Nam, H.S. Moon, K. Nam,
Incorporation of heavy metals bioavailability into risk characterization, Clean – Soil Air Water, 38 (2010) 812–815.
doi: 10.1002/clen.201000070
V. Tsiridis, M. Petala, P. Samaras, S. Hadjispyrou, G. Sakellaropoulos, A. Kungolos,
Interactive toxic effects of heavy metals and humic acids on Vibrio fischeri, Ecotoxicol. Environ. Safety, 63/1 (2006) 158–167.
doi:10.1016/j.ecoenv.2005.04.005
K. Lock, C.R. Janssen,
Influence of aging on metal availability in soils, Rev. Environ. Contam. Toxicol., 178 (2003) 1–21.
doi: 10.1007/0-387-21728-2_1
K. Vig, M. Megharaj, N. Sethunathan, R. Naidu,
Bioavailability and toxicity of cadmium to microorganisms and their activities in soil: a review, Adv. Environ. Res., 8 (2003) 121–135.
doi: 10.1016/S1093-0191(02)00135-1
T. Kunito, K. Saeki, H. Oyaizu, S. Matsumoto,
Influences of copper forms on the toxicity to microorganisms in soils, Ecotoxicol. Environ. Saf., 44 (1999) 174–181.
doi: 10.1006/eesa.1999.1820
K. Hughes, M.E. Meek, R. Newhook, P.K.L. Chan,
Speciation in Health Risk Assessments of Metals: Evaluation of Effects Associated with Forms Present in the Environment, Regul. Toxicol. Pharmacol., 22 (1995) 213-220.
 doi:10.1006/rtph.1995.0003
doi:10.1006/rtph.1995.0003 
S. Jonnalagadda,
Toxicity, bioavailability and metal speciation, Comp. Biochem. Physiol. C, 106/3 (1993) 585-595.
DOI: 10.1016/0742-8413(93)90215-7
C. B. Dissayanake,
Humic substances and chemical speciation - implications on environmental geochemistry and health, Int. J. Environ. Stud., 37/4 (1991) 247-258.
DOI: 10.1080/00207239108710637
M.L. Freedman, P.M. Cunningham, J.E. Schindler, M.J. Zimmerman,
Effect of lead speciation on toxicity, Bull. Environ. Contam. Toxicol., 25/1 (1980) 389-393.
DOI: 10.1007/BF01985543 Used Instrumentation:
Used Instrumentation:
 Shimadzu AA-7000 Flame atomic absorption spectrometer
Shimadzu AA-7000 Flame atomic absorption spectrometer Related EVISA resources
Related EVISA resources Brief summary: Speciation and Toxicity
Brief summary: Speciation and Toxicity Brief summary: The role of elemental speciation in legislation
Brief summary: The role of elemental speciation in legislation  Brief summary: Trace element speciation analysis for environmental sciences
Brief summary: Trace element speciation analysis for environmental sciences Related EVISA News
Related EVISA News
 February 20, 2014: New study shows: Coastal water, not sediment, predicts methylmercury bioaccumulation in the marine food web
February 20, 2014: New study shows: Coastal water, not sediment, predicts methylmercury bioaccumulation in the marine food web  February 15, 2011: Natural dissolved organic matter plays dual role in cycling of mercury
February 15, 2011: Natural dissolved organic matter plays dual role in cycling of mercury  August 19, 2007: Speciation and Toxicity: Humic Acids Increase Lead Bioavailability and Toxicity for Marine Invertebrates
August 19, 2007: Speciation and Toxicity: Humic Acids Increase Lead Bioavailability and Toxicity for Marine Invertebrateslast time modified: May 15, 2023