A group of researchers from Münster (Germany) found gadolinium in different brain regions of a patient post mortem with highest concentreations above 800 ng/g more than two years after having received a linear Gd-based contrast agent for diagnostic imaging.
Background:
Because of its highly paramagnetic properties, Gd is frequently used as a constituent of contrast agents in magnetic resonance imaging (MRI). Since Gd3+ ions are quiet toxic, free Gd cannot be applied for such purposes and therefore Gd is complexed with polyamino-carboxylic acid, forming stable chelates. Being applied in about 450 Mio. cases during the last 30 years, with few cases of severe side effects, Gd-based contrast agents are considered to be safe. The safety statement is based on the stability of the chelates meant to circumvent any transformation during the short residence time in the human body and the high water solubility allowing a fast fast excretion via the kidneys.
Anyhow, in 2006 reports appeared about a sporadic phenomenon called "nephrogenic systemic fibrosis" in patients with impaired renal function after GBCA application, showing Gd deposition in affected skin regions.
Regulatory authorities such as FDA and EMA reacted by changing the labeling of the agents with warning notes restricting the application for kidney impaired patients. Since then, researchers had a closer look onto the retention of Gd in the human body. As a result of research activities, since 2014 studies have shown, that MRI images of the brain of patients being more than once investigated, are showing a contrast effect resulting from former investigations. Such contrast enhancement was speculated to be the result of Gd retention in the brain, which has been meanwhile sunstantiated by analytical determination of Gd in the brain of autopsy samples by ICP-MS.
The new study:The researchers from Münster aimed at the development and application of an analytical method for the quantitative imaging of gadolinium in the human brain. By using laser ablation (LA) for spatially resolved sampling coupled to a highly sensitive inductively coupled plasma-mass spectromneter (ICP-MS) they obtained suitable limits of quantitation that allowed for a high spatial resolution.
Human brain samples came from the patients of the University Hospital Münster which had provided their consent to use their anonymized data for postmortem investigations prior to death, or the respective consent was given afterwards from corresponding relatives. Patient A died at an age of 64 and received an enhanced MRI examination using gadodiamide (16 mL, 0.5 mmol L.-1) 726 days before death. Patient B (62 years) did not receive any GBCA for MRI. Therefore, these samples served as a control. Although there were no respective data available indicating that, it cannot be excluded that patients received GBCAs at a much earlier stage at another medical institution. This unfortunately is general problem of retrospective studies on patients with limited history of medical treatments during their whole life.
The frozen autopsy brain samples were sectioned into slices of a thickness of 10 µm, mouinted on microscopic glass slides and stored at -20 °C until analysis by LA-ICP-MS.
Standards for external calibration of the instrument were prepared by gadolinium doped gelatin sliced with the same thickness as the samples and sampled under the same conditions as the samples. The calibration function was established over the wide range of 0.05 - 10 µg/g using 7 standards.
Using a 213-nm laser system (LSX-213, Teledyne CETAC) with a laboratory-build ablation chamber for improved transient behavior, operated at a laser spot size of 50 µn and a scanning speed of 100 µm/s, and a quadrupole based ICP-MS (Thermoe iCAP Qc), images were aquired by a line-by-line scanning method. Firing the laser with 20 Hz and an otimized laser energy of 1.46 J/Cm2, samples were ablated quantitatively. In norder to reduce polyatomic interferences on the isotopes of Gd (158Gd and 160Gd) and 103Rh used as internal standard) the ICP-MS was operated in KED mode with He as the cell gas. 31P was also determined as an ubiquitous element in human tissue helping tro visualize the tissue structure.
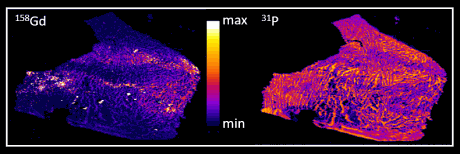
Figure: quantitative distribution maps for gadolinium and phosphorus of a human autopsy brain thin section investigated by LA-ICP-MS
The results obtained by LA-ICP-MS imaging revealed gadolinium deposits in different brain sections with highest concentrations above 800 ng/g more than two years after the last application of a GBCA. The limit of quantification was a factor of 100 lower, providing detection of gadolinium at concentrations much lower than those necessary for MRI enhancement effects. Since the Gd is present only at tzrace concentrations, a co-localization with phosphorous, present at much higher concentrations all over the tissue could not be observed. Therefore no information about the type of Gd-species being retained could be gained by this method.
With this technique, the authors have developed a method that is suitable to study the retention effect of different Gd-based contrast agents within different organs. Further studies will be necessary to differentiate effects of different classes of GBCAs and their mode of application, as well as the type of species being involved in short- and long-time retention. Methods suitable for such speciation analysis have been developed before by the research group of Prof. Uwe Karst (see the news below).
 The original study
The original study
Stefanie Fingerhut, Ann-Christin Niehoff,
Michael Sperling, Astrid Jeibmann, Werner Paulus, Thomas Niederstadt, Thomas Allkemper, Walter Heindel, Markus Holling,
Uwe Karst,
Spatially resolved quantification of gadolinium deposited in the brain of a patient treated with gadolinium-based contrast agents, J. Trace Elem. Med. Biol., 45 (2018) 125-130.
DOI: 10.1016/j.jtemb.2017.10.004 Homepage
of the research group of Prof. Karst at the Institute of Inorganic and
Analytical Chemistry of the University of Muenster (Germany)
Homepage
of the research group of Prof. Karst at the Institute of Inorganic and
Analytical Chemistry of the University of Muenster (Germany)
 Related studies (newest first)
Related studies (newest first)
D.R. Roberts, S.M. Lindhorst, C.T. Welsh, K.R. Maravilla, M.N. Herring, K.A. Braun, B.H. Thiers, W.C. Davis,
High levels of gadolinium deposition in the skin of a patient with normal renal function, Invest. Radiol., 51/5 (2016) 280–289.
DOI: 10.1097/RLI.0000000000000266
M. Birka, K.S. Wentker, E. Lusmöller, B. Arheilger, C.A. Wehe,
M. Sperling, R. Stadler,
U. Karst,
Diagnosis of nephrogenic systemic fibrosis by means of elemental bioimaging and speciation analysis, Anal. Chem., 87/6 (2015) 3321–3328.
DOI: 10.1021/ac504488k
T. Kanda, T. Fukusato, M. Matsuda, K. Toyoda, H. Oba, J. Kotoku, T. Haruyama, K. Kitajima, S. Furui,
Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy, Radiology, 276/1 (2015) 228–232.
DOI: 10.1148/radiol.2015142690
T. Kanda, K. Ishii, H. Kawaguchi, K. Kitajima, D. Takenaka,
High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material, Radiology, 270/3 (2014) 834–841.
DOI: 10.1148/radiol.13131669 L. Telgmann
L. Telgmann, C.A. Wehe, J. Künnemeyer, A.C. Bülter,
M. Sperling,
U. Karst, Speciation of Gd-based MRI contrast agents and potential products of transmetalation with iron ions or parenteral iron supplements, Anal. Bioanal. Chem., 404/8 (2012) 2133–2141.
DOI:
10.1007/s00216-012-6404-x
D.P. Hao, T. Ai, F. Goerner, X.M. Hu, V.M. Runge, M. Tweedle,
MRI contrast agents: basic chemistry and safety, J. Magn. Reson. Imaging, 36/5 (2012) 1060–1071.
DOI: 10.1002/jmri.23725
S. Aime, P. Caravan,
Biodistribution of gadolinium-based contrast agents, including gadolinium deposition, J. Magn. Reson. Imaging,30/6 (2009) 1259–1267.
DOI: 10.1002/jmri.21969
T. Frenzel, P. Lengsfeld, H. Schirmer, J. Hutter, H.J. Weinmann,
Stability of gadolinium-based magnetic resonance imaging contrast agents in human serum at 37 degrees C, Invest. Radiol., 43/12 (2008) 817–828.
DOI: 10.1097/RLI.0b013e3181852171
M.F. Tweedle, J.J. Hagan, K. Kumar, S. Mantha, C.A. Chang,
Reaction of gadolinium chelates with endogenously available ions, Magn. Reson. Imaging, 9/3 (1991) 409–415.
DOI: 10.1016/0730-725X(91)90429-P Related EVISA Resources
Related EVISA Resources
 Brief summary: Speciation analysis for the study of metallodrugs and their biomolecular interactions
Brief summary: Speciation analysis for the study of metallodrugs and their biomolecular interactions  Brief summary: ICP-MS - A versatile detection system for speciation analysis
Brief summary: ICP-MS - A versatile detection system for speciation analysis  Link Database: Toxicity of Gadolinium compounds
Link Database: Toxicity of Gadolinium compounds
 Link Database: Use of Gadolinium in pharmaceuticals
Link Database: Use of Gadolinium in pharmaceuticals
 Materials Database: Gadolinium Materials
Materials Database: Gadolinium Materials
 Link page: All about Mass Spectrometry: Resources related to Mass Spectrometry
Link page: All about Mass Spectrometry: Resources related to Mass Spectrometry  Related EVISA News
Related EVISA News
 July 29, 2017: EMA Issues Final Opinion Limiting Gadolinium Contrast Agents in Body Scans
July 29, 2017: EMA Issues Final Opinion Limiting Gadolinium Contrast Agents in Body Scans May 23, 2017: FDA identifies no harmful effects to date with brain retention of gadolinium-based contrast agents for MRIs
May 23, 2017: FDA identifies no harmful effects to date with brain retention of gadolinium-based contrast agents for MRIs  March 11, 2017: European Medicines Agency recommends to pull linear Gadolinium-based MRI contrast agents from market
March 11, 2017: European Medicines Agency recommends to pull linear Gadolinium-based MRI contrast agents from market  April 10, 2016: New Studies Question Safety of MRI Contrast Agents
April 10, 2016: New Studies Question Safety of MRI Contrast Agents  August 13, 2015: FDA investigating risk of gadolinium contrast agent brain deposits
August 13, 2015: FDA investigating risk of gadolinium contrast agent brain deposits March 4, 2015: Detection of Gd-based contrast agent in the skin of a patient eight years after administration
March 4, 2015: Detection of Gd-based contrast agent in the skin of a patient eight years after administration  October 29, 2012: Identification and quantification of potential metabolites of Gd-based contrast agents
October 29, 2012: Identification and quantification of potential metabolites of Gd-based contrast agents  September 15, 2010: US FDA Announces Gadolinium-Based MRI Contrast Agent Warning
September 15, 2010: US FDA Announces Gadolinium-Based MRI Contrast Agent Warning March 25, 2010: Publication on the separation of Gd-based contrast agents awarded
March 25, 2010: Publication on the separation of Gd-based contrast agents awarded May 4, 2009: Gadolinium speciation analysis in search for the cause of nephrogenic systemic fibrosis (NSF)
May 4, 2009: Gadolinium speciation analysis in search for the cause of nephrogenic systemic fibrosis (NSF) last time modified: October 16, 2017