A UK-based team of researchers combined an on-line sampling approach for soil solution with speciation analysis for hexavalent chromium. The temporal resolution of the approach is in the order of 15 min. and may be used to capture dynamic interactions.
Background:The differentiation of trivalent (Cr(III)) and hexavalent (Cr(VI)) chromium in soil and sediments is of great interest motivated by the significant differences in toxicity and mobility. The more toxic Cr(VI) species unfortunately is also more mobile and bioavailable in the soil-water systems compared to Cr(III) and is therefore also more likely to be transferred from contaminated soil to drainage water and into plants or aquifers.
The accurate determination of Cr(VI) in solid matrices such as soil or sediments is challenging due to the potential for species interconversion during extraction and analysis, especially when the sampling approach is disturbing the equilibrium within the sampled medium. The kinetics of Cr species interconversions in soil-pore water systems are of particular interest for understanding the transportation of Cr(VI) from contaminated soil into groundwater systems. To follow such processes with conventional sampling approaches produces large numbers of samples prone to artifactual errors.
The new study:
In order to overcome such problems and to follow interconversion of species more dynamically, a UK-based team of researchers propose to use microdialysis (MD) as a passive sampling approach for soil solution. The microdialysis system is using a probe having a semipermeable membrane with a specific molecular weight cut off; the continuous pumping of a perfusate solution into the probe creates a diffusion gradient within the sampled medium causing solutes to diffuse across the membrane. The solution exciting the probe (dialysate), containing the sampled chromium species is then analyzed by chromium speciation analysis. The researchers have developed a method using the hyphenation of MD with high performance liquid chromatography-inductively coupled plasma mass spectrometry (HPLC-ICP-MS) for this task.
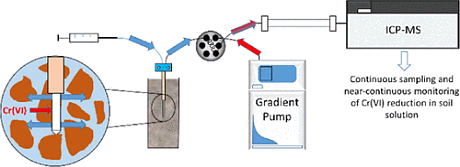
The optimization and calibration of the system was first performed by using stirred solution as sampled medium. A perfusate flow rate of 3 µL/Min was chosen as a compromise to obtain a high relative recovery of Cr(VI) from the samples medium of 55% and to allow a high temporal resolution of 15 min. The detection limit for the optimized conditions was 0.2 µg/L Cr(VI), a factor of 4 higher than for the direct HPLC-ICP-MS method. The calibration over the range 1-100 µg/L was highly linear.
The optimized method was then used for the analysis of soil solutions of three types of soil. The samples were chosen due to their total Cr(VI) content but also to provide a range of physicochemical properties to demonstrate the robustness of the approach. The system sampled and analyzed fresh soil solutions at 15 min intervals, offering improved temporal resolution and a significant reduction in analysis time over offline methods.
The authors also mention, that further improvements with respect to temporal resolution are possible by using more dedicated sample introduction systems such as low flow total consumption nebulizers. They also indicate that the approach could be used for other common inorganic species of interest.
 The Original study
The Original study
Elliott M. Hamilton, Scott D. Young, Elizabeth H. Bailey, Olivier S. Humphrey, and Michael J. Watts,
Online Microdialysis-High-Performance Liquid Chromatography-Inductively Coupled Plasma Mass Spectrometry (MD-HPLC-ICP-MS) as a Novel Tool for Sampling Hexavalent Chromium in Soil Solution, Environ. Sci. Technol., 55/4 (2021) 2422–2429.
DOI: 10.1021/acs.est.0c08140
 Related studies
Related studies

E.M. Hamilton, R.M. Lark, S.D. Young, E.H. Bailey, G.M. Sakala, K.K. Maseka, M.J. Watts,
Reconnaissance sampling and determination of hexavalent chromium in potentially-contaminated agricultural soils in Copperbelt Province, Zambia. Chemosphere, 247 (2020) 125984
DOI: 10.1016/j.chemosphere.2020.125984

S. Buckley, R. Brackin, S. Jämtgĺrd, T. Näsholm, S. Schmidt, Microdialysis in soil environments: Current practice and future perspectives. Soil Biol. Biochem., 143 (2020) 107743
DOI: 10.1016/j.soilbio.2020.107743

O.S. Humphrey, S.D. Young, E.H. Bailey, N.M.J. Crout, E.L. Ander, E.M. Hamilton, M.J. Watts,
Investigating the use of microdialysis and SEC-UV-ICP-MS to assess iodine interactions in soil solution. Chemosphere, 229 (2019) 41– 50,
DOI: 10.1016/j.chemosphere.2019.04.215

E
. Tassi, M. Grifoni, F. Bardelli, G. Aquilanti, S. La Felice, A. Iadecola, P. Lattanzi, G. Petruzzelli,
Evidence for the natural origins of anomalously high chromium levels in soils of the Cecina Valley (Italy). Environ. Sci.: Processes Impacts, 20 (2018) 965– 976,
DOI: 10.1039/C8EM00063H

C.R. Warren,
Development of online microdialysis-mass spectrometry for continuous minimally invasive measurement of soil solution dynamics. Soil Biol. Biochem., 123 (2018) 266– 275,
DOI: 10.1016/j.soilbio.2018.05.022

B. Palumbo-Roe, V.J. Banks, H.C. Bonsor, E.M. Hamilton, M.J. Watts,
Limitations on the role of the hyporheic zone in chromium natural attenuation in a contaminated urban stream. Appl. Geochem., 83 (2017) 108– 120,
DOI: 10.1016/j.apgeochem.2017.02.011

M.
Shahid, S. Shamshad, M. Rafiq, S. Khalid, I. Bibi, N.K. Niazi, C. Dumat, M.I. Rashid,
Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system: A review. Chemosphere 2017, 178, 513– 533,
DOI: 10.1016/j.chemosphere.2017.03.074

K. Matern, T. Mansfeldt,
Chromium Release from a COPR-Contaminated Soil at Varying Water Content and Redox Conditions. J. Environ. Qual., 45 (2016) 1259– 1267,
DOI: 10.2134/jeq2015.10.0506

J. Korf, K.D. Huinink, G.A. Posthuma-Trumpie,
Ultraslow microdialysis and microfiltration for in-line, on-line and off-line monitoring. Trends Biotechnol., 28 (2010) 150– 158,
DOI: 10.1016/j.tibtech.2009.12.005

Miró, M.; Fitz, W. J.; Swoboda, S.; Wenzel, W. W.
In-situ sampling of soil pore water: Evaluation of linear-type microdialysis probes and suction cups at varied moisture contents. Environ. Chem., 7 (2010) 123– 131,
DOI: 10.1071/EN09084

G. Jin, Q. Cheng, J. Feng, F. Li,
On-Line Microdialysis Coupled to Analytical Systems. J. Chromatogr. Sci., 46 (2008) 276– 287,
DOI: 10.1093/chromsci/46.3.276

M.
Miró, W. Frenzel, The potential of microdialysis as an automatic sample-processing technique for environmental research. TrAC, Trends Anal. Chem., 24 (2005) 324– 333,
DOI: 10.1016/j.trac.2004.10.004

M. Miró, M. Jimoh, W. Frenzel,
A novel dynamic approach for automatic microsampling and continuous monitoring of metal ion release from soils exploiting a dedicated flow-through microdialyser. Anal. Bioanal. Chem., 382 (2005) 396– 404,
DOI: 10.1007/s00216-004-3003-5

L.A. Hellerich, N.P. Nikolaidis,
Studies of hexavalent chromium attenuation in redox variable soils obtained from a sandy to sub-wetland groundwater environment. Water Res. 2005, 39, 2851– 2868,
DOI: 10.1016/j.watres.2005.05.003

M.
Miró, W. Frenzel,
Implantable flow-through capillary-type microdialyzers for continuous in situ monitoring of environmentally relevant parameters. Anal. Chem., 76 (2004) 5974– 5981,
DOI: 10.1021/ac049406a
 H. Ernstberger, W. Davison, H. Zhang, A. Tye, S. Young, Measurement and Dynamic Modeling of Trace Metal Mobilization in Soils Using DGT and DIFS. Environ. Sci. Technol., 36 (2002) 349– 354, DOI: 10.1021/es010917d
H. Ernstberger, W. Davison, H. Zhang, A. Tye, S. Young, Measurement and Dynamic Modeling of Trace Metal Mobilization in Soils Using DGT and DIFS. Environ. Sci. Technol., 36 (2002) 349– 354, DOI: 10.1021/es010917d
 M.P. Harper, W. Davison, H. Zhang, W. Tych, Kinetics of metal exchange between solids and solutions in sediments and soils interpreted from DGT measured fluxes. Geochim. Cosmochim. Acta, 62 (1998) 2757– 2770. DOI: 10.1016/S0016-7037(98)00186-0
M.P. Harper, W. Davison, H. Zhang, W. Tych, Kinetics of metal exchange between solids and solutions in sediments and soils interpreted from DGT measured fluxes. Geochim. Cosmochim. Acta, 62 (1998) 2757– 2770. DOI: 10.1016/S0016-7037(98)00186-0

S.M. Hassan, A.W. Garrison,
Distribution of chromium species between soil and porewater. Chem. Speciation Bioavailability, 8 (1996) 85– 103,
DOI: 10.1080/09542299.1996.11083273
last time modified: March 13, 2021